Course Review Slides
advertisement
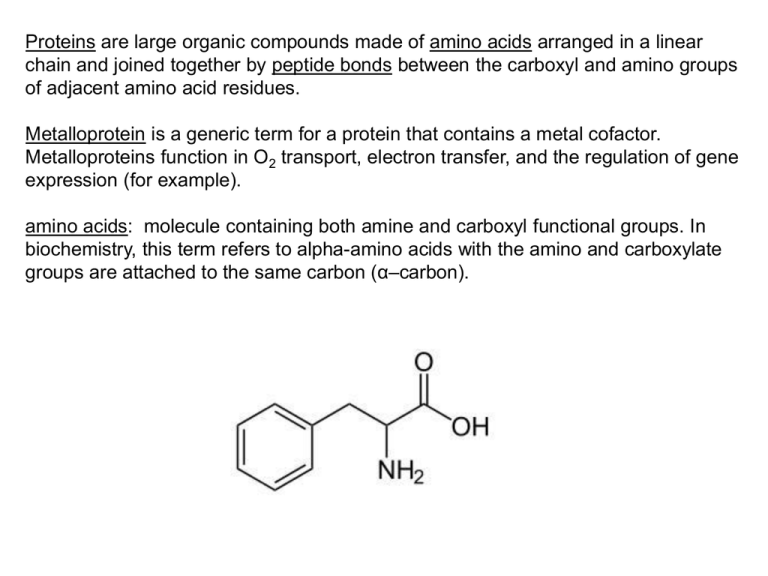
Proteins are large organic compounds made of amino acids arranged in a linear chain and joined together by peptide bonds between the carboxyl and amino groups of adjacent amino acid residues. Metalloprotein is a generic term for a protein that contains a metal cofactor. Metalloproteins function in O2 transport, electron transfer, and the regulation of gene expression (for example). amino acids: molecule containing both amine and carboxyl functional groups. In biochemistry, this term refers to alpha-amino acids with the amino and carboxylate groups are attached to the same carbon (α–carbon). primary structure secondary structure tertiary structure (or folding) Secondary Primary Tertiary quaternary structure Ligand Coordinate covalent bond Proteins as ligands: The most common amino acid groups that function as ligands are the thiolate of cysteine, the imidazole of histidine, the carboxylates of glutamic and aspartic acids and the phenolate group of tyrosine. Proteins as ligands: The most common amino acid groups that function as ligands are the thiolate of cysteine, the imidazole of histidine, the carboxylates of glutamic and aspartic acids and the phenolate group of tyrosine. tuning of redox potentials and electron transfer: electron transfer reactions are sensitive to both the ligand donor atoms and the stereochemistry at the metal centre. Example: Cu(I) adopts tetrahedral or trigonal geometries and prefers soft donor atoms. Cu(II) adopts square planar (or square pyramidal or octahedral) geometry and prefers harder donor atoms. By changing the ligand environment of a Cu(II) centre so that it is forced to distort into a near Td geometry through use of bulky ligands, or by introducing soft-ligand donors, Cu(II) can be more readily reduced to Cu(I) resulting in higher redox potentials. Ligand exchange rates: The anticancer drug cis-[Pt(NH3)2Cl2] (cisplatin) undergoes a ligand exchange process to lose a Cl- ligand and ultimately binds to DNA so strongly that only strong platinum binding agents, like CN- can displace the Pt-DNA bond. Subsequently, the platinum cross-links two bases via displacement of the other chloride ligand. Inner-sphere vs. outer-sphere electron transfer Myoglobin and Hemoglobin Porphyrin MbO2 and HbO2 adducts are Fe(III) complexes with the superoxide ligand, O2-. Coordination of dioxygen to deoxy Hb or Mb is accompanied by electron transfer to form O2-, which is stabilized by hydrogen bonding to a distal imidazole (His) proton, and coupling between O2- and Fe(III) leads to a diamagnetic, S = 0, ground state. Allosteric Effect proximal histidine Allosteric Effect The Fe-O-O bond angle is 115o, therefore the bond is “bent”. Allosteric Effect X-ray (and for myoglobin) neutron diffraction studies indicate the formation of a hydrogen bond between the coordinated dioxygen molecule and the N-H proton of the distal histidine residue. Allosteric Effect Three primary methods for synthesizing Fe model complexes are: 1. Synthesizing porphyrins that are sterically crowded on the distal side 2. Coordination of a sterically hindered axial base as a mimic for the T-state. 3. Attaching the five-coordinate system to a surface, like silica gel, to reduce its mobility and ability to add a sixth ligand. Cytochrome: Any of a class of iron-containing proteins important in cell respiration as catalysts of oxidation-reduction reactions. Cytochrome P450: one of a large group of iron-containing oyxgenases that utilize atmospheric dioxygen to functionalize molecules using cofactors such as flavins or NAD(P)H, as well as non-heme iron or copper and other metalloporphyrin complexes. Structure and Function: There are two main functional roles for these oxygenases. 1. The metabolism of xenobiotics (compounds exogenous to the organism). 2. The biosynthesis of critical signaling molecules used for control of development and homeostasis. Cytochrome c (Cyt c): can be defined as electron transfer proteins having one or several heme c groups, bound to the protein by one or two thioether bonds involving cysteine residues. The fifth, axial heme iron ligand is always provided by a histidine residue. Cyt c possess a wide range of properties and function in a large number of different redox processes. Iron-sulfur proteins: characterized by the presence of iron-sulfur clusters containing two, three or four iron centres that are sulfide-linked with variable oxidation states. The Fe centres are generally tetrahedral. The thiolato sulfur centers are from cysteinyl residues. The sulfide groups are either two- or three-coordinate. Rubredoxin: low-molecular-weight iron-containing proteins found in sulfurmetabolizing bacteria and archaea; electron transfer between [Fe(III)(Cys)4]1- and [Fe(II)(Cys)4]2-. Aconitase: metalloenzyme that catalyses the stereo-specific isomerization of citrate to isocitrate via cis-aconitase in the second step of the citric acid (Krebs) cycle, a non-redox-active process (proceeds by the elimination or addition of water). Aconitase has an active [Fe4S4]2+ cluster, which may convert to an inactive [Fe3S4]+ form. ferredoxin: iron-sulfur proteins that mediate electron transfer in a range of metabolic reactions. The metallo-sites can be of the form Fe2S2, Fe3S4 or Fe4S4. Nitrogenase: complex enzyme composed of two components. The MoFe protein contains all of the machinery to perform the reaction, but requires a steady source of electrons. The reaction requires the addition of six electrons for each nitrogen molecule that is split into two ammonia molecules. The Fe protein uses the breakage of ATP to pump these electrons into the MoFe protein. Ferritins: act in metal storage. Transferrin: acts in metal transport. Superoxide Dismutase (SOD): enzymes that disproportionate the biologically harmful superoxide ions that leak from respiratory enzymes and are harmful to cells. M(n+1)+SOD + O2− → Mn+SOD + O2 Mn+SOD + O2− + 2H+ → M(n+1)+SOD + H2O2 2O2- + 2H+ H2O2 + O2 CuZnSOD Plastocyanin: a copper-containing mobile electron-carrier protein involved in electron-transfer between photosystem II and I in higher plants and some algae. Azurin: small copper containing metalloenzyme involved in photosynthetic and respiratory electron transfer. Hemocyanin: large, multisubunit proteins capable of transporting oxygen, found in various arthropods and mollusks O2 + 2Cu(I) O22- + 2Cu(II) Ascorbate oxidase: a homodimeric, copper-containing enzyme which catalyzes the redox reaction between ascorbate and oxygen in plants and bacteria. Carbonic anhydrases: a family of zinc-containing metalloenzymes that catalyze the rapid conversion of carbon dioxide to bicarbonate and protons in tissues where there is a high CO2 concentration. Carboxypeptidase A: zinc-containing metalloenzyme that hydrolyzes the peptide bond adjacent to the C-terminal end of a polypeptide chain. Cisplatin: cis-diamminedichloridoplatinum(II); platinum-based chemotherapy drug Vitamin B12: cobalt and corrin ring containing molecules involved in DNA synthesis and regulation, fatty acid synthesis and energy production.











