LH1 - MyCourses
advertisement

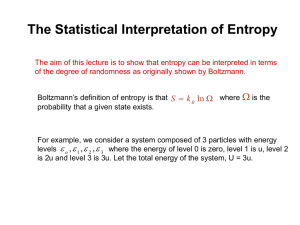
NBE-E4100 Molecular biophysics Exercise 1 January 20 2016 In this exercise we revive our basic knowledge of probability and statistical physics LH1-1The probabilities of identical sequences of amino acids. You are comparing protein amino acid sequences for homology. You have a 20-letter alphabet (20 different amino acids). Each sequence is a string n letters in length. You have one test sequence and s different data base sequences. You may find any one of the 20 different amino acids at any position in the sequence, independent of what you find at any other position. Let p represent the probability that there will be a ‘match’ at a given position in the two sequences. (a) In terms of s, p, and n, how many of the s sequences will be perfect matches (identical residues at every position)? (b) How many of the s comparisons (of the test sequence against each database sequence) will have exactly one mismatch at any position in the sequences? LH1-2 The combinatorics of disulfide bond formation. A protein may contain several cysteines, which may pair together to form disulfide bonds as shown in the figure below. If there is an even number n of cysteines, n/2 disulfide bonds can form. How many different disulfide pairing arrangements are possible? This disulfide bonding configuration with pairs 1–6, 2–5, and 3–4 is one of the many possible pairings. Count all the possible pairing arrangements. LH1-3. Calculating the entropy of mixing. Consider a lattice with N sites and n green particles. Consider another lattice, adjacent to the first, with M sites and m red particles. Assume that the green and red particles cannot switch lattices. This is state A. (a) What is the total number of configurations WA of the system in state A? (b) Now assume that all N +M sites are available to all the green and red particles. The particles remain distinguishable by their color. This is state B. Now what is the total number of configurations WB of the system? Now take N = M and n = m for the following two problems. (c) Using Stirling’s approximation, what is the ratio WA/WB? (d) Which state, A or B, has the greatest entropy? Calculate the entropy difference given by W DS = S A - S B = k ln W A . B LH1-4 The free energy and entropy of membrane melting. Pure membranes of dipalmitoyl lecithin phospholipids are models of biological membranes. They melt at Tm = 41◦C. Reversible melting experiments indicate that ΔHm = 9 kcal mol−1. Calculate (a) the entropy of melting ΔSm, and (b) the free energy of melting ΔGm. (c) Does the membrane become more or less ordered upon melting? (d) There are 32 rotatable CH2–CH2 bonds in each molecule. What is the increase in multiplicity on melting one mole of bonds? LH1-5 Lattice model of ligand binding to a protein. You have a ligand L and a protein P. When the ligand binds, the energy change is favorable by 5ε0 (ε0 < 0) because it makes 5 contacts with the protein. As shown below, the proteinis modeled using two-dimensional lattice particles. The protein has a rigid portion (squares containing a P) and a flexible loop (circles containing a P) at one end. The ligand (rectangle containing an L) is rigid and occupies two lattice sites. (a) Counting only lateral contacts (no diagonal connections), find the number of conformers W of the flexible loop. (b) What are the energetic and entropic contributions to the free energy change due to ligand binding? Write an expression for ΔFbinding. (c) For T = 300 K, what is ΔFbinding = Fbound − Funbound if ε0 = −1 kcal mol−1? (d) What is the dissociation temperature Tdissoc? (e) If the flexible loop were made rigid instead (and in the proper configuration for ligand binding), what would be the new ΔFbinding? (f) Does the ligand bind more tightly or more weakly with the rigid loop?