Lecture 9
advertisement

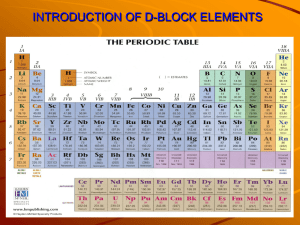
Regulations for American Pupils and Middle School Students • • • • • • • • • Always refer to a teacher by title and last name Get to class on time Raise your hand when you want to ask a question You may speak to the teacher from your desk while you are seated When you are absent, you must make up the work you have missed. Ask either the teacher or a classmate for the work If you expect to be away from school because of an emergency, tell your teacher in advance and ask for the work you will miss All assignments you hand in must be your own work Never cheat on a test ... 1 Transition elements: the facts I Transition elements: the facts I DEFINITIONS d block element: any element with its highest energy electron in a d orbital Transition element: those elements having ions with electrons in an incomplete d shell i.e. from titanium to copper 2 Transition elements: the facts I TYPICAL PHYSICAL PROPERTIES: all metals high m.p. chromium 2160K, iron 1800K compared with sodium 371K hard dense but not titanium similar atomic and ionic sizes and ionization energies. Unlike elements in the s and p blocks, there is little change in atomic and ionic radii as the d block is crossed. This is because the additional electrons are going into an inner d sub-shell. This also results in only a small increase in ionization energy across the d block. Although each successive nucleus has one more proton, this extra positive charge is partly shielded from the outer 4s electrons by the extra d electron in an underlying shell. 3 Transition elements: the facts I TYPICAL CHEMICAL PROPERTIES 1. Variable valency Transition elements show many oxidation states; these fall into two kinds: ● higher oxidation states: the covalently bonded oxo-compounds e.g. CrO42-; Cr2O72-; MnO4-; MnO42● lower oxidation states: the atomic ions e.g. Cr3+; Cr2+; Mn3+; Fe3+; Fe2+; Cu2+; Cu+ 4 Transition elements: the facts I 2. Colored compounds Many of the compounds of the transition elements are colored. common examples: CrO42--yellow; Cr2O72--orange; Cr3+-green; Cr2+-blue; chromate(VI) dichromate MnO4--purple; MnO42--green; Mn2+-pale pink (per)manganate(VII) manganate (VI) Fe3+-yellow; Fe2+-green Co2+-pink in water, blue when dry Cu2+-blue ● 5 Transition elements: the facts I 3. Catalytic properties Transition metals and their compounds can be: heterogeneous catalysts, for example: iron in the Haber process V2O5 in the Contact process ● homogeneous catalysts, for example: Mn2+ in the reaction between ethanedioate and manganate(VII) Fe2+/Fe3+ in the reaction between iodide ions and peroxydisulphate (VI). ● 6 Transition elements: the facts II Transition elements: the facts II Magnetic properties Some of the transition elements are ferromagnetic which means that they can be magnetized, e.g. iron, cobalt, and nickel. Some of their compounds are paramagnetic which means that they move in a strong magnetic field. 7 Transition elements: the facts II TYPICAL CHEMICAL PROPERTIES 4. Complex ion formation A complex ion consists of a central ion or atom surrounded by other particles called ligands. A ligand is a particle (ion or molecule) with a lone pair which forms a dative covalent bond to the central particle. The ligands are said to be coordinated to the central particle. Transition metal ions form many complex ions which vary in charge, shape, color, and stability. 8 Transition elements: the facts II Shape Ligands differ in size and this means that the number which can fit around the central cation changes. Ammonia and water are relatively small ligands and six of each can fit around a cobalt or copper ion forming octahedral complexes, while only four of the larger chloride ion can fit around either the cobalt or copper ions. Silver is unusual in forming linear complexes. [NC—Ag – CN][H3N—Ag –NH3]+ [O3S2—Ag – S2O3]39 Transition elements: the facts II Charge The charge of the complex depends on the relative charges of the central ion or atom and the ligands, and on the number of ligands around it. Complex ions may be cations or anions cationic complexes anionic complexes Cu(NH3)4(H2O)22+(aq), CuCl42-(aq), FeCNS2+(aq) Fe(CN)63-(aq) Color The color of the complexes is affected by the nature of the ligand and the number of ligands around the central cation. 10 Transition elements: the theories I Transition elements: the theories I PHYSICAL PROPERTIES The physical properties are dominated by the fact that the electrons with the highest energies go into an inner 3d orbital rather than the outer 4s orbital. These electrons in an underlying d orbital increase the electron repulsion on the outer 4s electrons. The elements are hard and have high m.p.s because they have high lattice energies. The lattice energies are high because the effective nuclear charge of the cations in the lattice is high, because the electrons in the d orbitals are bad at shielding the nuclear charge. 11 Transition elements: the theories I The sizes of the atoms and ions do not decrease much as the block is crossed. Although the atoms of each successive element have one more proton in the nucleus, increasing the attraction on the outer 4s electrons, there is increased repulsion on these outer electrons caused by the new electron in the inner d shell. The first ionization energies do not increase very much for the same reason. There are more protons attracting the electrons as one crossed the d block, but also more inner electrons repelling the outer electrons. The two effects almost cancel out. 12 Transition elements: the theories I CHEMICAL PROPERTIES 1. Variable valencies In higher oxidation states the transition elements form molecules and molecule ions because of the availability of vacant d and p orbitals, which can accept electrons from the surrounding atoms. The lower oxidation states happen because the transition elements have successive ionization energies which are similar in value to the size of hydration energies. This is not the case with the s block metals. e.g. compare sodium and iron 13 Transition elements: the theories I Sodium Iron So the hydration energy in solutions (and the lattice energy in solids) makes up for the slightly higher ionization energies in the higher oxidation states of iron, but not the very much higher ionization energies in the likes of sodium. 14 Transition elements: the theories I 2. Color Light falling on transition element compounds interacts with the d electrons. Some of the wavelengths in the light are absorbed leaving the complementary colors to be seen. 15 Transition elements: the theories I 3. Catalytic properties Heterogeneous catalysis The fact that the d block elements have 3d as well as 4s electrons helps them to form bonds with gaseous particles and so adsorb them onto the catalyst surface. This adsorption weakens the bonds in the gas particle, so lowering the activation energy of the reaction (see page 41 on catalysis). Homogeneous catalysis The fact that d block elements can exist in so many oxidation states is a crucial factor in making them such good homogeneous catalysts (see page 41 on catalysis) 16 Transition elements: the theories II Transition elements: the theories II CHEMICAL PROPERTIES 4. Complex formation d electrons are not as good at shielding the positive charge of the nucleus as either s or p electrons. This means that transition metal atoms and ions have greater polarizing power than the atoms and ions from the s block. The poorly shielded nucleus attracts lone pairs of electrons strongly and dative covalent bonds are formed between the central atom and the ligands, forming a complex ion—or complex for short. 17 Transition elements: the theories II There is often more than one kind of ligand in a solution and, if so, the different ligands will compete for the cation. For example, aqueous ammonia contains both water and ammonia ligands. The better a ligand is at competing for cation, the more stable will be the complex formed. A stable complex has a large Kstab value, so a table of Kstab figures allows you to predict whether one ligand will replace another. e.g. Kstab(Cu(NH3)42+) = 1.4×1013dm12mol-4 but Kstab(CuCl42- ) = 4×105dm12mol-4 So ammonia ligands will replace chloride ligands around copper if the concentrations of the two are the same. 18 Transition elements: the theories II* Chelates A ligand with more than one lone pair can form more than one dative bond with the central atom if the lone pairs are the right distance apart. Because a ligand with two lone pairs looks rather like a claw, it is called a chelate from the Greek wordmeaning claw. If the ligand has two lone pairs it is called a bidentate chelate. Hemoglobin(血色素) contains a tetradentate chelate while the ion ethanediaminotetraethanoate (acetate) is a hexdentate chelate known by its initials EDTE(EDTA). 19 20 Transition elements: the facts and theories words Words and Expressions Titanium; vanadium; chromium; manganese; iron; cobalt; nickel; copper; zinc ionic radius; ionic radii octahedral; tetrahedral; linear ferromagnetic; paramagnetic vacant orbitals; filled orbitals; incomplete orbitals heterogeneous catalysis; homogeneous catalysis adsorption; adsorb; absorb; absorption chelate; claw bidentate; tetradentate; hexadentate Hemoglobin(血色素) 21