Ions Powerpoint
advertisement
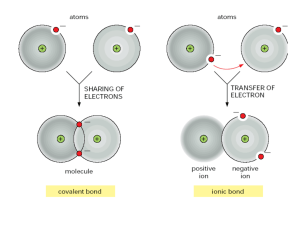
Ions Wandering electrons • In the last few weeks, we talked about the balance between protons and electrons in a neutral atom. Atomic Model Reminders 1 H 1.00794 1p+ e- Wandering electrons • In the last few weeks, we talked about the balance between protons and electrons in a neutral atom. • What happens if we have an electron that leaves an orbital? What happens to that atom? Atomic Model Reminders 1 H 1.00794 1p+ e- Well, if the electron left our hydrogen atom… Then the hydrogen atom would have a positive charge because of the single proton remaining. So our hydrogen atom would become an ion and be written: H+ Ions • A neutral atom that loses or gains electrons is called an ion. • Ions are particles with an overall positive or negative charge. • Let’s try a few more! Atomic Model Reminders 3 Li 6.941 3p+ 4n0 2e- 1e- + Li Let’s say an electron was to leave this atom of lithium… How many positive charges would you have? How many negative charges would you have? What is the total charge on the ion? How would you write the new symbol? Atomic Model Reminders 12 Mg 24.3050 12p+ 12n0 2e- 8e- 2e- 2+ Mg Let’s say two electrons were to leave this atom of magnesium… How many positive charges would you have? How many negative charges would you have? What is the total charge on the ion? How would you write the new symbol? Atomic Model Reminders 9 F 18.9984 9p+ 10n0 2e- 7e 8e- F Let’s say an electron was to be added to this atom of fluorine… How many positive charges would you have? How many negative charges would you have? What is the total charge on the ion? How would you write the new symbol? 1e- Why do electrons do this? • Valence orbital = Outermost orbital • Atoms are the most stable when they have full valence energy levels. • It takes energy to move these electrons from place to place. So electrons will move in whatever way is the least costly. • Electrons will transfer in a direction which requires the least moves to make complete valence orbitals. Atomic Model Reminders 11 Na 22.9898 17 Cl 35.453 11p+ 12n0 2e- 8e- 1e- 7e 8e- - 8e- 2e- 17p+ 18n0 + Na Cl Let’s say an electron was transferred between these two atoms, where would it go? Notice how many valence electrons each ion now has. How many positive charges would each ion have? How many negative charges would each ion have? What is the total charge on the ion? How would you write the new symbol? Ionic Compounds • Many compounds are formed from ions. • Most ions try to have 8 electrons in their valence shell (except H and He). This is called the “rule of eight” or “octet rule” • Ionic compounds stay together because oppositely charged ions are attracted to each other. • When compounds are formed this way, we say that they have an ionic bond. Ions in Solution • When ionic compounds break apart we say that they “dissociate”. • Some pairs of ions break apart in a solution. In a strong acid, nearly all ions separate in water. While in weak acids, few ions break apart. How do you know? • When an element is likely to give up or capture electrons, we say that it is “reactive”. • Reactive elements are generally the most dangerous, because they are looking to exchange electrons, which is usually associated with forming new compounds and often releasing energy! Uses of Ions • Although ionic compounds serve a variety of uses, one of the most useful is the storage and transfer of electrical energy. • Ions in solution carry electrical charge in water. • Ions are also used to store electricity in most batteries.