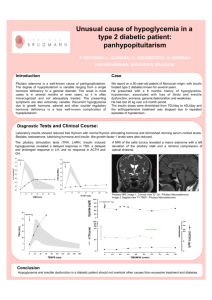
Human Growth Hormone
advertisement

Human Growth Hormone CHEE450 By Leslie Davis Human Growth Hormone Simulates linear growth in humans Effects on the growth plates of the long bones Increased cell growth Increased protein, carbohydrate and lipid metabolism Human Growth Hormone Generally used to treat those with GHdeficiencies Beneficial effects: burns, bone fractures and Turner’s syndrome Shown promise in treating chemotherapy and AIDS Increases body mass Human Growth Hormone Polypeptide 191 amino acid residues MW = 22125 daltons Highly hydrophobic proteins Historical Perspective Isolated hGH in human pituitary 1956 Proof of effectiveness 1958 Obtained from human pituitary gland only Urgent need to obtain human pituitary glands at autopsy High cost Cloned from human gene in 1980s Recombinant DNA Synthesis of hGH Cloning of human DNA Adapting of the cloned gene for expression in Escherichia coli E. coli did not have the biochemical machinery 84 base pairs of ds DNA synthesized for direct expression Cloning of the gene How it works Chemical messenger produced by the pituitary gland Pituitary Gland Promotes growth during childhood Plays important metabolic role throughout adulthood Production Process Fermentation Highly reproducible Intracellular E. coli W3110 with a pB322-derived plasmid coding Antibiotic resistance of genetically modified E. coli strain Typically tetracycline and ampicillin resistant Production Process Glucose and mineral salt medium Exponentially growing cells in batch mode Transferred from seed fermentor to production bioreactor 10% of final volume High cell density fermentation Production Process Fed-batch bioreactor Glucose feed ~ 500-600 g/L May use peptone for amino acid source Temperature – near maximum tolerance of E. coli Agitation generally ranges 1000-1500 rpm Slightly positive pressure Production Process Purification Cell disruption Microfiltration Chromatographic methods Genentech adapted anion exchange chromatography and gel filtration technology (early 80s) Production Process Issues with scale up Mass transfer through high density cells Oxygen Glucose feed Inhibitory ion formation Acetate and formate Final Product White lyophilized powder Low oral bioavailability (<1%) Intended for intravenous, subcutaneous or intramuscular administration Sold in vials or cartridges containing 4 - 24 mg of somatropin Preservatives Water for injection Final Product Approximately 28 day shelf life Oxygen Sensitive Must be refrigerated Improve the solubility, sodium dodecyl sulfate (extremely surface active) or denaturing agents - urea and guanidine hydrochloride Market U.S. Manufacturers Genotropin by Pharmacia & Upjohn Company, Humatrope by Eli Lilly, Norditropin by Novo Nordisk, Saizen and Serostim by Serono Laboratories, Nutropin by Genentech Market Costs: $75 to $200 per week World-wide sales of $1.6 billion Approximately 20,000 U.S. children taking drug Prescribed for non-GH-deficient children if (U.S.) <3SD below mean for age category <25% growth velocity Discussion Psychological issues Cultural “heightism” Increased metabolism, reduced fat, anti-aging, gain strength Articles References Human Growth Hormone, edited by Salcatore Raiti and Robert A. Tolman, 1986 Human Growth Hormone: Research and Clinical Practice, edited by Roy G. Smith and Michael O. Thorner Influence of Scale-Up on the Quality of Recombinant Human Growth Hormone, Bylund, Castan, Mikkola, Veide and Larsson Modelling the effects of Glucose Feeding on a recombinant E. coli Fermentation, Cockshott and Bogle Further Studies Related to the Scale-up of High Cell Density Escherichia coli Fed-Batch Fermentations: The Additional Effect of a Changing Microenvironment When Using Aqueous Ammonia to Control pH References http://pi.lilly.com/us/humatrope-pi.pdf http://www.hghnews.us/p/91.html http://www.hghhumangrowthhormone.com/hgh_human_growth _hormone_sitemap/hgh_manufacturer.html http://www.novonordisk.com/therapy_areas/grow th_hormone_therapy/default.asp http://patft.uspto.gov/netacgi/ http://www.genotropin.com/patients/growth_disor ders/index.asp