Acids and Bases (Part B)
advertisement

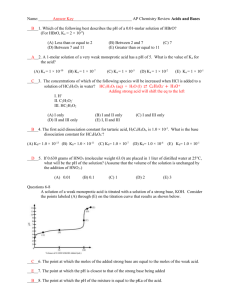
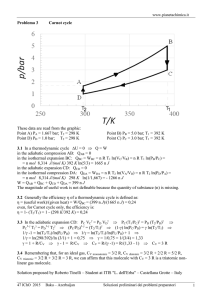
Elise Hyser and Amanda Homan Acids and Bases Neutralization Strong Bases Strong Acids Weak Bases Weak Acids pH Titration Curves Titration Curve: A graph of pH (of an acid or base) as a function of the volume of base (or acid) added to neutralize the substance Important Terms and Concepts to Know: -Equivalence Point: Point in a titration where the added solute reacts completely with the solute present in the solution. -Ka=Acid-Dissociation Constant -Kb=Base-Dissociation Constant -pH=-log[H+] -pKa=-log[Ka] -Kb=1.0x10-14 / Ka -ALWAYS WRITE OUT CHEMICAL EQUATIONS WHEN COMPLETING A TITRATION PROBLEM! 1. ) Solution with weak acid+strong base 4.) Use Ka,, [HX] , and [X-] to calculate [H+] 2.) HX+OH- 5.) Arrive at pH X-+H2O 3.) Calculate [HX] and [X-] after rxn Calculation of a solution’s pH when 45.0 mL of .100 M NaOH is added to 50.0 mL of .100 M HC2H3O2 (Ka=1.8x10-5): .0500L x (.100 mol HC2H3O2 /1L soln) =5.00x10-3 mol HC2H3O2 .0450L x (.100 mol NaOH /1L soln) =4.50x10-3 mol NaOH Total volume of solution: 45.0mL +50.0mL=.0950L [HC2H3O2 ] =.50x10-3 mol /.0950L =.0053 M [C2H3O2-] = 4.50x10-3 mol /.0950L=.0474 M Ka= [H+] [C2H3O2-] / [HC2H3O2 ] =1.8x10-5 [H+]= Ka x[ [HC2H3O2 ] / [C2H3O2-]] =2.0x10-6M pH=-log[2.0x10-6]=5.70 Solubility of Compound (g/L) Molar Concentration of Ions Molar Solubility of Compound (mol/L) Ksp (Solubility Product Constant) http://www.chemguide.co.uk/physical/acidbaseeqia/summary.gif www.sparknotes.com H2SeO3 + H2O == H3O+ +HSeO3HSeO3- +H2O == SeO32- + H3O+ SeO32- +H2O == HSeO3- + OH- Solution involving the weak acid=higher initial pH than solution of a strong acid of equal concentration pH change at “rapid-rise” portion of curve near equivalence point is smaller for weak acid than it is for strong acid pH at equivalence point is over 7.00 for weak acid-strong base titration Thymol Blue 1.2-2.8 Methyl yellow 2.9-4.0 Methyl orange 3.1-4.4 Methyl red 4.4-6.2 Phenol red 6.4-8.0 Phenolphthalein 8.0-10.0 Alizarin yellow 10.0-12.0 pH Meter: Electronic instrument used to measure the acidity or basicity of a liquid (consists of a glass electrode & electronic meter) Potentiometric Titration: “A volumetric method in in which the potential between two electrodes is measured as a function of the added reagent volume. Types of potentiometric titrations include acid-base, redox, precipitation, and complexometric” (Chemistry Dictionary & Glossary). Involves determining concentration of a solution by reacting it with a certain # of moles of excess reagent. Excess reagent is later titrated with another reagent. Source: diracdelta.co.uk The Common Ion Effect: The extent of ionization of a weak electrolyte is decreased by adding a strong electrolyte to the solution that has an ion in common with the weak electrolyte. 0.10 M HC2H3O2 solution has a pH of 2.9, while a solution containing 0.10 M HC2H3O2 and 0.01 M NaHC2H3O2 has a pH of 4.7. Why has the addition of NaHC2H3O2 to a HC2H3O2 solution caused a decrease in H+ concentration (increase in pH)? When C2H3O2- (from the strong electrolyte NaC2H3O2) is added to an acetic acid solution at equilibrium, the equilibrium condition is displaced. According to LeChatelier’s principle, the system reestablishes equilibrium by removing some of the added acetate ion. HC2H3O2 (aq) Equilibrium H+(aq) + C2H3O2-(aq) C2H3O2- is added shifts left to remove some C2H3O2- This simultaneously removes H+ ions from solution with the formation of HC2H3O2 and thereby increases the pH. Solutions containing a weak acid and its • conjugate base or a weak base and its conjugate acid Used to control the pH of a solution Because of the common ion effect the mixture of a weak acid and its conjugate base inhibits the ionization of both A buffer also reduces changes in pH due to the addition of a strong acid or strong base. Strong acid added to a buffer reacts with the conjugate (weak) base to form more of the conjugate (weak) acid. Strong base added to a buffer reacts with the conjugate (weak) acid to form more of the conjugate (weak) base. Ka - [H ] [: A ] [ HA ] log K a log ( - [H ] [: A ] ) [ HA ] - log K a log[H ] log [HA] By Definition - log [K a ] pK - log[H Therefore ] pH : - pK a [A ] pH log www.tiem.utk.edu [A ] [HA] a What is the change in pH when 0.010 mol solid NaOH is added to 1.0L of a buffered solution containing 0.50M acetic acid (HC2H3O2, Ka = 1.8 x 10-5) and 0.50M sodium acetate (NaC2H3O2) with a pH of 4.74? Before reaction: After reaction: Initial: Change: Equilibrium: HC2H3O2 0.50 mol 0.49 mol + OH0.010 mol 0 HC2H3O2 (aq) == 0.49 -x 0.49 - x H+ (aq) + 0 x x == C2H3O2- + H2O 0.50 mol 0.51 mol C2H3O2- (aq) 0.51 x 0.51 + x Ka = 1.8 x 10-5 = [H+][C2H3O2-] / [HC2H3O2] = (x) (0.51 + x) / (0.49 - x) [H+] = x = 1.7 x 10-5 M pH = 4.76 The change in pH from adding 0.010 mol OH- to the buffered solution is only: 4.76 - 4.74 = +0.02 The amount of acid or base the buffer can neutralize before the pH begins to experience a noticeable change Depends on the amount of acid and base from which the buffer is made The greater the amounts of the conjugate acid-base pair, the more resistant the ratio of their concentrations (and therefore pH) is to change