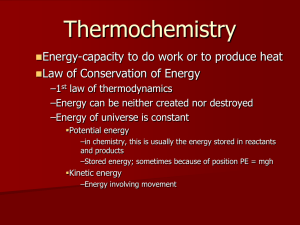
The Rate of Chemical Reactions
advertisement
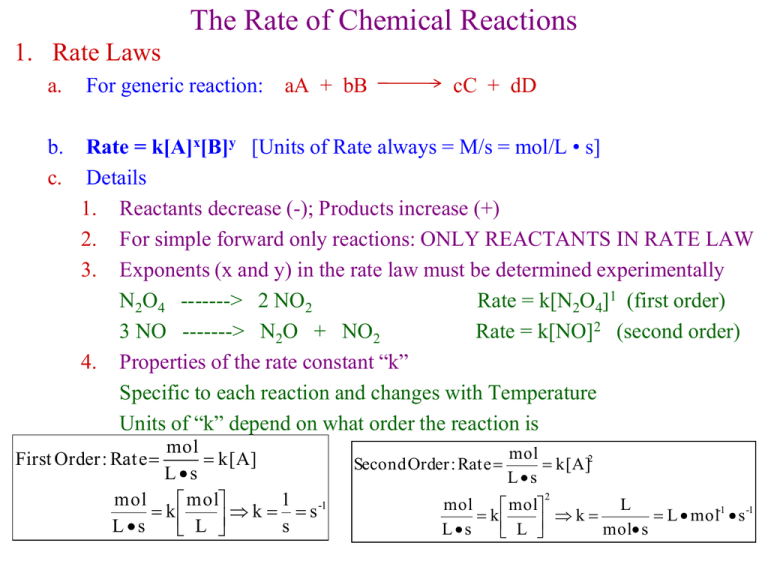
The Rate of Chemical Reactions 1. Rate Laws a. For generic reaction: aA + bB b. c. Rate = k[A]x[B]y [Units of Rate always = M/s = mol/L • s] Details 1. Reactants decrease (-); Products increase (+) 2. For simple forward only reactions: ONLY REACTANTS IN RATE LAW 3. Exponents (x and y) in the rate law must be determined experimentally N2O4 -------> 2 NO2 Rate = k[N2O4]1 (first order) 3 NO -------> N2O + NO2 Rate = k[NO]2 (second order) 4. Properties of the rate constant “k” Specific to each reaction and changes with Temperature Units of “k” depend on what order the reaction is mol k[A] Ls mol 1 -1 mol k k s Ls L s First Order : Rate cC + dD Second Order : Rate mol k[A]2 Ls 2 mol L mol k k L mol-1 s -1 Ls mol s L 2. Method of Initial Rates a. b. Method for determining the “order” of the reactants (exponents in rate law) First order reactants = [A]1 Double the reactant concentration -------> Doubles the rate of reaction [A]exp 2 [A]exp 1 2 Rateexp 2 Rateexp 1 2 (Conc.Ratio) Rate Ratio c. First Order Second order reactants = [A]2 Double the reactant concentration -------> quadruples the rate of reaction [A]exp 2 Rateexp 2 2 4 [A]exp 1 Rateexp 1 (Conc.Ratio) Rate Ratio d. 21 2 2 2 4 Second Order Zero order reactants = [A]0 (changing concentration has no effect on rate) [A]exp 2 Rateexp 2 2 1 [A]exp 1 Rateexp 1 (Conc.Ratio) Rate Ratio 20 1 ZeroOrder 3. Temperature, Reaction Rates, and the Arrhenius Equation k = rate constant A = frequency factor (combines z and p) Ea = activation energy T = temperature in Kelvins R = gas constant = 8.3145 J/K.mol a. Taking the natural log of each side gives us another form of the equation that gives a linear plot. lnk vs. 1/T gives straight line with slope = -Ea/R and intercept = ln(A) Ea ln(k) R 1 ln(A) T b. Example: 2 N2O5 4 NO2 + O2 Ea? T(oC) T(K) 1/T(K) k (s-1) ln(k) 20 293 3.41x10-3 2.0x10-5 -10.82 30 303 3.30x10-3 7.3x10-5 -9.53 40 313 3.19x10-3 2.7x10-4 -8.22 50 323 3.10x10-3 9.1x10-4 -7.00 60 333 3.00x10-3 2.9x10-3 -5.84 Ea E a (slope)(R) -(-1.2x 104 K)(8.3145J/mol K) R E a 99,774 J/mol 100 kJ/mol slope 4. Reminder about the Dilution Equation Example: 5 ml of 0.015 M KIO3 is added to 2.5 ml HSO3- and 7.5 ml H2O M1V1 M 2 V2 M2 M1V1 (0.015M)(5ml) 0.005M KIO3 V2 (15 ml) 5. Today’s Reactions: KIO3 + NaHSO3 rate = k[IO3-]n[HSO3-]m IO3- + 3HSO3- -------> I- + 3SO42- + 3H+ IO3- + 8I- + 6H+ -------> 3I3- + 3H2O I3- + HSO3- + H2O -------> 3I- + SO4- + 3H+ fast fast slow Starch + I3- -------> Colored Complex a) The “slow” reaction occurs until all of the HSO3- is gone, then Blue color forms b) We will determine the rate of the “slow reaction” by timing how long it takes for the Blue Complex to appear. c) Rate = ([HSO3-]initial)/(time till Blue) = [0.0125 M]/73s = 1.72 x 10-4 mol/L•s 6. Reminder: a) Experiments 1-5 at different concentrations give us Rate Law and k b) Experiments 6-9 at different temperatures give us Ea