CommonIonEffectAndBuffers_Printable
advertisement

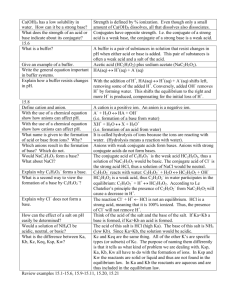
Chem 112 Class Guide: COMMON ION EFFECT AND BUFFERS Chapter 17, Sections 1 and 2 Learning Goals: Upon completion of Chapter 16, Sections 6-9, you should be able to determine the following: Calculate the pH of a solution made up of two components that contain one common ion Describe a buffer Calculate the pH of a buffer solution Chapter Reading Guide: Chapter 17, Sections 1 and 2 Section 1: THE COMMON ION EFFECT Read Chapter 17.1 When a weak electrolyte and a strong electrolyte are placed into solution together, the weak electrolyte will ionize less than if it were alone in solution. If you have two acids, one strong and one weak, the pH of the solution will be based upon the concentration of H+ at equilibrium (remember that the weak acid will reach equilibrium). When setting up this ICE chart, you will have initial concentrations of both the weak acid AND H+ (coming from the ionization of the strong acid). Solve for x, calculate your equilibrium H concentration and then calculate pH. Example: Calculate the pH of a solution that is 1.5 M HCl and 1.5 M HC2H3O2. The Ka of HC2H3O2 is 1.8x10-5. HCl is a strong acid, so it ionizes completely in solution: HCl H+ + Cl-. That means the concentration of H+ and Cl- are 1.5M. HC2H3O2 is a weak acid and is in equilibrium: HC2H3O2 ↔ H+ + C2H3O2-. We need to calculate H+ using an ICE chart: HC2H3O2 H+ C2H3O2Initial 1.5 1.5 (from HCl) 0 Change -X +X +X Equilibrium 1.5 – X 1.5 + X X H C2 H 3O2 1.5 X X 1.5 X Ka 1.8 x105 , so X 1.8 x105. HC2 H 3O2 1.5 X X Equilibrium [H+] = 1.5 + 1.8x10-5 = 1.5 M pH = -log[H+]=-log(1.5) = 4.74 If you are dealing with two solutions, remember that when they mix, they dilute each other. The dilution formula (M1V1 = M2V2) may need to be used in order to calculate the postmixing concentrations of each acid. These concentrations are then used in the ICE chart. Try Practice exercises 17.1 and 17.2 Section 2: BUFFERED SOLUTIONS Read Chapter 17.2 A buffer solution consists of a weak electrolyte and its conjugate. Most often, they are weak bases and their conjugate acids. In order to calculate the pH of a buffer solution, you should use the Henderson-Hasselbalch equation: [ BASE ] pH pK a log [ ACID] Example: Calculate the pH of a buffer that is 1.5 M HC2H3O2 and 2.0 M C2H3O2-. The Ka of HC2H3O2 is 1.8x10-5. pKa = -log(Ka)=-log(1.8x10-5) = 4.74 2.0 [ BASE ] pH pK a log 4.74 0.125 4.87 4.74 log [ ACID] 1.5 The buffering capacity of a buffer is the amount of acid/base that a buffer can absorb before the buffer significantly changes pH. The more acid/base that is in a buffer, the greater the buffering capacity of the buffer is. Try Practice exercises 17.3 and 17.4 Learning Resources Chapter Learning Goals Chapter 17, Sections 1 and 2 Learning Goals Pre Class Assignment: This assignment must be completed prior to the next class. Check your syllabus for the exact due date and time. Complete to the pre class assignment (http://berks.psu.edu/clt/chem112/CommonIonEffectAndBuffers_HW.docx) Submit a copy to the dropbox located in ANGEL called “Pre Class Assignment Submission: Common Ion Effect and Buffers” End of Chapter Problems: Practice with these problems if you are having difficulty with any of the concepts covered in this class guide AFTER we have met in class. If you cannot easily complete these problems, seek help from your instructor, your mentor or the learning center Chapter 17: 15, 19, 21