Group 7 (VII) - Miller, Jonathan
advertisement

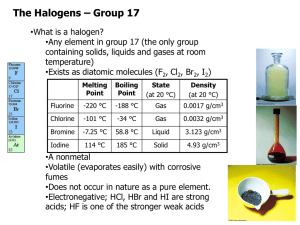
Chapter 6 The Periodic Table: Group7 6.1 The Halogens 6.2 Reactions of The Halogens and Their Ions 6.1 What is the outcome from syllabus? Candidates should be able to: ♣ describe the trends in volatility and colour of chlorine, bromine and iodine ♣ interpret the volatility of the elements in terms of van der Waals’ forces ♣ describe the relative reactivity of the elements as oxidising agents ♣ describe and explain the reactions of the elements with hydrogen ♣ describe and explain the relative thermal stabilities of the hydrides and their relative stabilities in terms of bond energies 6.1 What is the outcome from syllabus? All the elements in Group 7 are nonmetals except for astatine, which is a radioactive metalloid. These elements are called halogens, which means “salt-former.” All of the halogens form salts with sodium and with the other alkali metals. 6.1 The Halogens greenish gas red-brown liquid black solid 6.1 The Halogens Covalent radius/nm Tm/K Tb/K Cl 0.099 172 238 Br 0.114 266 332 I 0.133 387 457 The melting and boiling temperatures increase as going down the group, because larger atoms makes the van der Waals forces between the molecules stronger. 6.1 The Halogens + e- X halogen → Xˉ reduction halide F Cl Br Increasing oxidising power This is because the electronegativity increases as we go up the group, which means that the elements gain electrons more easily. I For example: Cl2 (aq) + 2Brˉ (aq) → Br2 (aq) + 2Clˉ(aq) 6.1 The Halogens 6.1 The Halogens Formation of hydrogen hylides with hydrogen H2(g) + Cl2 (g) hν or heating HCl (g) vigorously reaction H2(g) + F2 (g) hν or heating HF (g) hard to control H2(g) + Br2 (g) H2(g) + I2 (g) hν or heating hν or heating rather slowly, almost no reactions 6.1 The Halogens Laboratory preparations of HX CaF2 + H2SO4(conc.) → CaSO4 + 2HF(g) NaCl + H2SO4(conc.) → NaHSO4 + HCl(g) ∵ 2HBr(g) + H2SO4(conc.) → SO2(g) + 2H2O(g) + Br2(l) 8HI(g) + H2SO4(conc.) → H2S(g) + 4H2O(g) + 4I2(l) ∴ NaBr(s) + H3PO4(l) → NaH2PO4(aq) + HBr(g) NaI(s) + H3PO4(l) → NaH2PO4(aq) + HI(g) 6.1 The Halogens Acidity of hydrogen hylides in aqueous solution HF H2O Hydrofluoric acid Hydrogen fluoride HCl H2O Hydrogen chloride HBr H2O Hydrogen bromide HI Hydrogen iodide The strong H—F bond must be broken to release H+ H2O Weak acid Hydrochloric acid Hydrobromic acid Hydroiodic acid Strong acid 6.1 The Halogens Thermal Stability of HX Bond Tabulated bond energy/kJ mol-1 H-F 568 H-Cl 432 H-Br 366 H-I 298 2HX(g) → H2(g) + X2(g,l,s) By plunging a red-hot wire into a test tube of the gas: HI: easily decomposed. HBr: may or may not decompose depending on the exact temperature of the wire. HCl and HF: not decomposed. 6.1 The Halogens √ √ 6.2 What is the outcome from syllabus? Candidates should be able to: ♣ describe and explain the reactions of halide ions with aqueous silver ions followed by: (i) aqueous ammonia (ii) concentrated sulphuric acid ♣ outline a method for the manufacture of chlorine from brine by a diaphragm cell ♣ describe and interpret in terms of changes of oxidation number the reaction of chlorine with cold, and with hot, aqueous sodium hydroxide ♣ explain the use of chlorine in water purification ♣ recognise the industrial importance and environmental significance of the halogens and their compounds, (e.g. for bleaches; PVC; halogenated hydrocarbons as solvents, refrigerants and in aerosols) 6.2 Reactions of the Halogens and Their Ions 6.2 Reactions of the Halogens and Their Ions Test for halide ions The presence of Cl-(aq), Br-(aq) and I-(aq) can be confirmed by adding a few drops of silver nitrate solution (fluorides are soluble): AgNO3(aq) + X-(aq) AgX(s) + NO3-(aq) Silver halide Colour Chloride White Bromide Cream Iodide Yellow Silver chloride and bromide dissolve in concentrated ammonia, but the iodide does not. AgBr(s) + 2NH3(aq) [Ag(NH3)2]+(aq) + Br-(aq) 6.1 Reactions of Halogens and their ions Reactions of halide ions with conc. H2SO4 CaF2 + H2SO4(conc.) → CaSO4 + 2HF(g) NaCl + H2SO4(conc.) → NaHSO4 + HCl(g) With bromides and iodides, a redox reaction occurs: NaBr(s) + H2SO4(conc.) → NaHSO4 + HBr(g) 2HBr(g) + H2SO4(conc.) → SO2(g) + 2H2O(g) + Br2(l) NaI(s) + H2SO4(conc.) → NaHSO4 + HI(g) 8HI(g) + H2SO4(conc.) → H2S(g) + 4H2O(g) + 4I2(l) 6.2 Reactions of the Halogens and Their Ions Colours of Silver halides Ag+(aq) + Cl-(aq) Ag+(aq) + Br-(aq) Ag+(aq) + I-(aq) AgCl(s) AgBr(s) AgI(s) 6.2 Manufacture of Chlorine from brine An Anode (+): 2Cl-(aq) At Cathode (-): 2H2O(l) + 2e- Cl2(g) + 2e- 2OH-(aq) + H2(g) Overall: 2NaCl(aq) + 2H2O(l) Cl2(g) + H2(g) + 2NaOH(aq) 6.2 Reactions of Halogens and their ions Reactions of chlorine with Alkalis Cold dilute alkali: Cl2(g) + 2NaOH(aq) Cl-(aq) + ClO-(aq) + H2O(l) Hot concentrated alkali: 3Cl2(g) + 6NaOH(aq) 5Cl-(aq) + ClO3-(aq) + H2O(l) Both examples of disproportionation Cl-: Cl (-I) Cl2: Cl (0) ClO-: Cl (+I) ClO3-: Cl (+V) (Roman numerals: I = 1; V = 5) 6.2 Reactions of Halogens and their ions Reactions of chlorine with Alkalis Cold dilute alkali: Cl2(g) + 2NaOH(aq) Cl-(aq) + ClO-(aq) + H2O(l) Hot concentrated alkali: 3Cl2(g) + 6NaOH(aq) 5Cl-(aq) + ClO3-(aq) + H2O(l) Both examples of disproportionation Cl-: Cl (-I) Cl2: Cl (0) ClO-: Cl (+I) chlorate(I) ClO3-: Cl (+V) chlorate(V) (Roman numerals: I = 1; V = 5) 6.2 Reactions of Halogens and their ions Commercial uses of halogens and their compounds Chlorine is used in water purification as it destroys harmful bacteria that could accumulate in old and unrecycled drinking water. Sodium chlorate(I), NaClO(aq) is used in bleaches. Halogenated hydrocarbons are used as solvents (dichloromethane CH2Cl2 dissolves many organic compounds). CFCs were used as refrigerants and aerosols; section 12.3 They do not contain hydrogen atoms! Some are used as anaesthetics: Fluothane - CF3CHBr.