File
advertisement
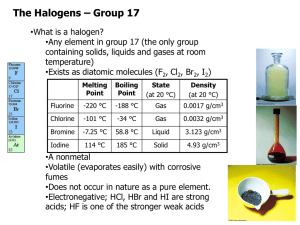
2.7 Inorganic chemistry of group 7 (limited to chlorine, bromine and iodine) Cro2012 Group 7 – the halogens The elements in group 7 of the periodic table, on the right, are called the halogens. F fluorine Cl chlorine Br bromine I At iodine astatine a. recall the characteristic physical properties of the elements limited to the appearance of solutions of the elements in water and hydrocarbon solvents In water: In hydrocarbon solvents: • Chlorine solution is pale green • Bromine solution is yellow to orange • Iodine solution is brown. • Chlorine solution is pale green • Bromine solution is yellow to orange • Iodine solution is pink-purple. Oxidation Reaction of Halogens b. describe and carry out the following chemical reactions of halogens: i. oxidation reactions with metal and non-metallic elements and ions such as iron(II) and iron(III) ions in solution With Metals: React strongly with electropositive metals, removing the outer electrons to become reduced themselves. Oxidation Reaction of Halogens b. describe and carry out the following chemical reactions of halogens: i. oxidation reactions with metal and non-metallic elements and ions such as iron(II) and iron(III) ions in solution With Non-Metals: The halogen usually achieves a noble gas configuration by forming a covalent bond: Reactivity decreases Oxidation Reaction of Halogens b. describe and carry out the following chemical reactions of halogens: i. oxidation reactions with metal and non-metallic elements and ions such as iron(II) and iron(III) ions in solution With Iron(II) chloride solution: Disproportionation b. describe and carry out the following chemical reactions of halogens: ii. disproportionation reactions with cold and hot alkali, eg hot potassium hydroxide with iodine to produce potassium iodate(V) Give the oxidation numbers for the chlorine containing species. Disproportionation b. describe and carry out the following chemical reactions of halogens: ii. disproportionation reactions with cold and hot alkali, eg hot potassium hydroxide with iodine to produce potassium iodate(V) Iodine/thiosulfate titration c. carry out an iodine/thiosulfate titration, including calculation of the results and evaluation of the procedures involved, eg determination of the purity of potassium iodate(V) by liberation of iodine and titration with standard sodium thiosulfate solution Remember that when carrying out this titration the starch is added near the end point (when the solution is pale yellow). Addition of starch to a solution that contains iodine or triiodide ion forms a reversible blue complex. The disappearance of this blue coloured complex is a much more sensitive method of determining the end point. However, if the starch is added to a solution which contains a great deal of iodine, the complex which forms may not be reversible. Therefore, the starch is not added until shortly before the end point is reached. Reaction with conc. Sulphuric Acid d. describe and carry out the following reactions: i. potassium halides with concentrated sulfuric acid, halogens and silver nitrate solution White steamy fumes • With sodium bromide/iodide, the hydrogen halides are oxidised by the acid: Orange/brown fumes or colour Purple fumes and/or black solid Displacement (Redox) Reactions d. describe and carry out the following reactions: i. potassium halides with concentrated sulfuric acid, halogens and silver nitrate solution • Potassium halides react with other halogens: • What gets reduced and what gets oxidised? A more reactive halogen will displace a less reactive halogen from it’s halide in solution. So: • Cl2 displaces Br2 from Br- and I2 from I• Br2 displaces I2 from I- d. Testing for Halides: Silver Halides describe and carry out the following reactions: i. potassium halides with concentrated sulfuric acid, halogens and silver nitrate solution ii. silver halides with sunlight and their solubilities in aqueous ammonia solution Addition of dilute nitric acid Then add aqueous silver Nitrate solution. Testing for Halides: Silver Halides Equations Partial decomposition turns AgCl into grayish precipitate in sunlight. d. describe and carry out the following reactions: ii. silver halides with sunlight and their solubilities in aqueous ammonia solution The silver halides are unstable in the presence of sunlight. They decompose forming silver (seen as dark specs) and the halogen, for example: 2AgI(s) 2Ag(s) + I2(s) Reaction of Hydrogen Halides d. describe and carry out the following reactions: iii. hydrogen halides with ammonia and with water (to produce acids) • Hydrogen halides react with ammonia gas to form ammonium halides: • Hydrogen chloride dissolves in water to form hydrochloric acid. e. make predictions about fluorine and astatine and their compounds based on the trends in the physical and chemical properties of halogens. What are the general properties of the halogens? All the halogens are: Diatomic non-metals and so do not conduct electricity brittle and crumbly when solid poisonous and smelly. Very reactive and strong oxidising agents. They become darker in colour down the group: is pale yellow is yellow-green is red-brown is grey What is the electron structure of the halogens? All halogens have seven electrons in their outer shell. This means that: They can easily obtain a full outer shell by gaining one electron. They all gain an electron in reactions to form negative ions with a -1 charge. They have similar chemical properties. fluorine 2,7 chlorine 2,8,7 bromine 2,8,8,7 The reactivity of alkali metals decreases going down the group. What is the reason for this? The atoms of each element get F larger going down the group. This means that the outer shell gets further away from the nucleus and is shielded by more electron shells. Cl The further the outer shell is from the positive attraction of the nucleus, the harder it is to attract another electron to complete the outer shell. This is why the reactivity of the halogens decreases going down group 7. Br decrease in reactivity How does electron structure affect reactivity? What is the physical state of the halogens? The melting and boiling points of the halogens increase down the group, as the molecules become bigger. Halogen Relative size Melting point (°C) Boiling point (°C) State -220 -118 gas -101 -34 gas -7 59 liquid 114 184 solid What is the state of each halogen at room temperature? Halogen Solubility • Solubility in water decreases down group. • Chlorine reacts in water to form chlorine water ( a mixture of HCl and chloric (I) acid: • Chloric (I) acid is what gives a solution of chlorine its bleaching properties. • Bromine reacts in a similar way, but to a lesser extent.