Neutralization and Titration
advertisement

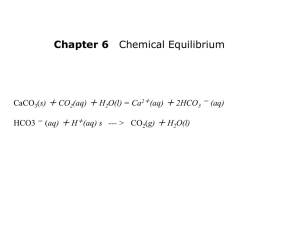
Acid + Base Salt + Water Salt = ionic compound made up of the anion from an acid and the cation from a base. REMEMBER: when any strong acid reacts with any strong base in the mole ratio from a balanced chemical equation, a neutral aqueous solution of a salt is formed. Note: reactions between acids and bases of different strengths usually don’t result in neutral solutions. 1. pH probe 2. Indicator An indicator is usually a weak monoprotic acid with a complex organic structure. HIn(aq) ↔ H+(aq) + In-(aq) Phenolphthalein: Color 1 Color 2 (You do not need to know this structure) What are they? How do they work? Antacids are supposed to decrease the amount of hydrochloric acid in the stomach by reacting with excess acid (ie. they neutralize your stomach acid). They are used in the treatment of gastric hyperacidity and peptic ulcers. Some of the ingredients in antacids are: Magnesia (MgO), milk of magnesia (Mg(OH)2, calcium carbonate (CaCO3), sodium bicarbonate (NaHCO3), dihydroxyaluminum sodium carbonate (NaAl(OH)2CO3), aluminum hydroxide gel (Al(OH)3). What’s going to happen when I put the TUMS (main ingredient is CaCO3) in the acid? What if I add phenolphthalein? What if I add bromythol blue? What if I add bromocresol green? What volume of 0.250 M H2SO4(aq) is needed to react completely with 37.2 mL of 0.650 M KOH(aq)? Step 1: Balanced equation H2SO4(aq) + 2KOH(aq) K2SO4(aq) +2H2O(l) Step 2: amount (mols) of KOH= 0.650 mol/L x 0.0372 L = 0.02418 mol KOH Step 3: mols H2SO4 (reacts with KOH in 1:2 ratio)= 0.02418 mol KOH x ( ) = 0.01209 mol H2SO4 Step 4: Volume H2SO4(aq) = = 0.04836 L Therefore, the volume of H2SO4(aq) required is 48.4 mL. NOW: If you are more comfortable working with this equation, go ahead! Just be careful with your mole ratios! 2CAVA = CBVB H2SO4(aq) What volume of 0.150 M hydrochloric acid is needed to neutralize 80.0 mL of 0.0045 M calcium hydroxide? Moving On… It’s a method chemists use to determine the concentration of a solution by observing it’s quantitative reaction with a solution of known concentration In other words, if we have some volume of acid and we want to determine it’s concentration, we can “titrate” it with a solution of base of which we know the concentration (or vice versa) Titrant: what we put in the buret Endpoint = point at which your indicator (just) changes color Graph of the pH of an acid (or base) vs the volume of titrant base (or acid) You need to know what these types of titration curves look like: Strong acid + strong base Weak acid + strong Base Weak base + strong acid You do not need to know the curve for the titration of a weak acid with weak base Why? It’s complicated! Strong acid + strong base titrations: equivalence point is always pH 7 Want an indicator that has an endpoint close to the equivalence point Equivalence point is greater than pH 7 Equivalence point is less than pH 7 http://www.mhhe.com/physsci/chemistry/a nimations/chang_7e_esp/crm3s5_5.swf http://wwwchem.uwimona.edu.jm:1104/sof tware/titr.html Assignment for the remainder of class: In groups of 2-3, go online and find one of the following: An article relating in acids and/or bases An application (industrial, medicinal, environmental,etc.) of acids/bases A cool experiment relating to acids or bases Prepare to give us the “gist” of what you discover in 2-3 minutes Need help? You could look at: Natural buffer systems Acid-base chemistry and baking Acid rain Use of indicators (ex. medicine) Natural acid-base remedies (ex. bee/wasp stings) Acidic or basic drugs extracted from natural sources