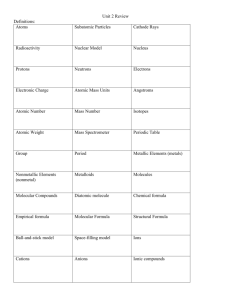
AP Chemistry
advertisement
AP Chemistry Unit 3 - Elements Lesson 4 – Isotopes Book Section: 2.3 Isotopes Isotopes are atoms of the same element with different masses. Isotopes have different numbers of neutrons. 11 6 C 12 6 C 13 6 C 14 6 C Atomic Mass Atomic and moleculear masses can be measured with great accuracy with a mass spectrometer. Average Mass Because in the real world we use large amounts of atoms and molecules, we use average masses in calculations. Average mass is calculated from the isotopes of an element weighted by their relative abundances. Sample Problem Naturally occurring chlorine is 75.78% 35Cl, which has an atomic mass of 34.969 amu, and 24.22% 37Cl, which has an atomic mass of 36.966 amu. Calculate the average atomic mass (that is, atomic weight) of chlorine. Sample Problem Naturally occurring chlorine is 75.78% 35Cl, which has an atomic mass of 34.969 amu, and 24.22% 37Cl, which has an atomic mass of 36.966 amu. Calculate the average atomic mass (that is, atomic weight) of chlorine. 35.45 amu AP Problem A) B) C) D) E) 1989 MC #24 The mass of element Q found in 1.00 mole of each of four different compounds is 38.0 grams, 57.0 grams, 76.0 grams, and 114 grams, respectively. A possible atomic weight of Q is 12.7 19.0 27.5 38.0 57.0 AP Problem A) B) C) D) E) 1989 MC #24 The mass of element Q found in 1.00 mole of each of four different compounds is 38.0 grams, 57.0 grams, 76.0 grams, and 114 grams, respectively. A possible atomic weight of Q is 12.7 19.0 – 64% correct, easy 27.5 38.0 57.0 AP Problem 1984 MC #19 Which of the following represents a pair of isotopes? Atomic Number A) B) C) D) E) Mass Number I. II. I. II. I. 6 7 6 14 6 14 14 7 14 14 II. 14 28 I. II. I. 7 7 8 13 14 10 II. 16 20 AP Problem 1984 MC #19 Which of the following represents a pair of isotopes? Atomic Number A) B) C) D) E) Mass Number I. II. I. II. I. 6 7 6 14 6 14 14 7 14 14 II. 14 28 I. II. I. 7 7 8 13 -- 87% correct 14 – very easy 10 II. 16 20 HW: 2.22, 2.24, 2.26, 2.28, 2.30, 2.32, 2.34 This Week: Tuesday – Periodic Table Structure (2.4, 7.1) Wednesday – Quantitative Analysis of a Soluble Sulfate Day 2 Thursday – Wave-Particle Duality (6.1, 6.2) Friday – Gravimetric Analysis of a Chloride Salt Lab 10/7 – Quantitative Analysis of a Soluble Sulfate Due 10/18 – Gravimetric Analysis of a Chloride Salt Due 10/20 – Elements Exam 10/21 – Problem Set 2 Due