Unit 1: METABOLIC PROCESSES - Emery
advertisement

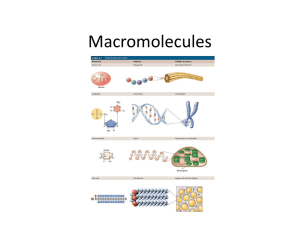
GRADE 12 BIOLOGY (SBI4U) MACROMOLECULES Macromolecules: What you need to know! 2 1. Structure of the basic unit (carbohydrates, lipids, proteins, nucleic acids) 2. How they react to form larger molecules 3. How the larger molecules are broken down into basic units 4. Functions of the molecules in living organisms What is a … 3 Polygons Polyester Polygamy 4 Polymers • long molecules • have many similar or identical repeating building blocks (structural units, monomers, small molecules) • connected by chemical bonds (covalent bonds) 5 Monomers • The smallest repeating unit of a polymer • Can exist individually 6 7 Macromolecules • Organic molecules constructed of smaller units called polymers – these polymers are subdivided into their basic units called monomers + + + 8 Macromolecules • a macromolecule is also called … biological macromolecule … biomolecule … organic molecule … large carbon-carbon molecule to name a few… 9 Macromolecules • fall into 4 major categories – can you name them? Hint: 3 of the 4 can be found in foods! 10 Macromolecules: 4 major categories 11 Macromolecules: Question: Which one isn’t considered a polymer and why? 12 13 Macromolecules: Why do we care? • Macromolecules are the molecules of life! • How do you build a cell? • Start with water, add lots of small carbon-containing molecules and ……. use these four major classes of macromolecules 14 Macromolecules • All living things are made of cells • Cells are: ~72% H2O ~3% salts (Na, Cl, K…) ~25% carbon compounds (macromolecules) 15 Macromolecules Ions, small molecules (4%) Lipids (2%) Nucleic Acids (DNA and RNA (1% + 6%) Proteins (15%) Carbohydrates (2%) 16 Making and Breaking of Polymers or “Condensation” or “Dehydration” Synthesis (aka polymerization) why synthesis? – a polymer grows in length (a new bond is made) why dehydration (or condensation) – formation of a water molecule “Addition” polymerization monomer molecules added to a growing polymer chain NO molecules are eliminated in the process monomer is unsaturated (e.g., had a double bond) after an addition reaction it becomes saturated Hydrolysis (Cleavage) hydrolysis (hydro = water, lysis = break) – reverses the process of dehydration by breaking down the polymer with the addition of water molecules Carbohydrates (sugar/starch) • Monosaccharide (b/w 3-7 carbon atoms) - Contain multiple hydroxyl groups and a carbonyl group – the simplest sugars glucose fructose, galactose ribose deoxyribose 21 - Contains C, H, O in ratio of 1:2:1 Isomers Isomers – one of two or more molecules with the same number and type of atoms, but different structural arrangements e.g. glucose, fructose, galactose - Also differ in chemical and physical properties 22 Carbohydrates • Disaccharides – 2 simple sugars (sucrose, lactose = glucose + galactose, maltose = glucose + glucose) - Bond linking monosaccarides together = glycosidic linkage 23 Carbohydrates • Polysaccharides (‘complex’) – many sugars (e.g. starch, cellulose, glycogen, chitin) – energy storage – structural materials glycogen 24 cellulose Carbohydrates (polysaccharides) 25 Note the linking of simple repeating units Carbohydrates (polysaccharides) 26 Lipids • • • • • fatty acids hydrocarbons comprised of fatty acids hydrophobic reservoirs of energy structural materials – cell membrane • 4 forms of lipids – neutral fats, phospholipids, sterols, waxes 27 Lipids – Neutral fats • neutral fats – three fatty acids and a glycerol – body’s most abundant lipid • functions – energy reservoir – insulation 28 Fats glycerol + 3 fatty acid fat (triglyceride) Ester linkage Fats Animal vs. Plant Fats Animal Fats Plant Fats - Triglycerols containing mostly saturated fatty acids -triglycerols are unsaturated and polyunsaturated FA - Straight hydrocarbon chains allowing for van der Waal attractions -Hydrocarbon chains have double bonds and many kinks, ↓ van der Waal attractions - Solid at room temperature - Liquid at room temperature Fatty Acids saturated fat unsaturated fat trans-unsaturated fat Lipids – Neutral fats • Can be used for insulation adipose tissue 33 Lipids - Phospholipids • form double-layered cell membranes 34 Phospholipids Glycerol backbone + 2 fatty acids + phosphate phospholipid Phospholipids Phospholipids have: 1. a hydrophobic head 2. a hydrophobic tail Due to the dual chemical nature of the molecule, it is said to be amphipathic. Phospholipids Phospholipids Phospholipid Bilayer Sterols also known as steroids Proteins Proteins are used for: structure metabolism (enzymes) immunological protection molecular transport Proteins are made of subunits of amino acids. Proteins are the most diverse class of macromolecules due to 20 available amino acids. Amino Acids Amino Acids in Aqueous Solutions • Amino acids contain a basic amine group, which can act as a proton acceptor, and an acidic carboxylic acid group, which can act as a proton donor Amino Acids Essential vs. Non-essential Amino Acids Essential Amino Acids: Cannot be produced by the body, therefore must be consumed in ones diet 8 essential Amino Acids Non-essential Amino Acids: Can be produced by the body 13 Non-essential Amino Acids Peptides amide bond Protein Organization Four layers of protein organization: 1. 2. 3. 4. primary (1°) structure secondary (2°) structure tertiary (3°) structure quaternary (4°) structure Primary (1°) Structure sequence of amino acids polypeptide chain Second (2°) Structure H-bond between peptide bonds 1. a-helix 2. b-pleated sheets not necessarily in all proteins Second (2°) Structure Tertiary (3°) Structure provides protein a final 3-D structure four major bond types between R groups of amino acids 1. 2. 3. 4. H-bonding ionic bonding hydrophobic interactions covalent bond (disulfide bridge) Tertiary (3°) Structure General Protein Shapes Globular Proteins (Hemoglobin) Fibrous Proteins (Tropomyosin & Keratin) Quaternary (4°) Structure fully functional protein requires all subunits present not all proteins have quaternary structure Protein Properties Proteins have optimal conditions at which they function. When exposed to extreme conditions, proteins begin to unfold – denature. If denaturation occurs moderately over time, returning to the original conditions may result in renaturation. Protein Folding Proper folding in the cell is completed by chaperonin molecules. Utilizes ATP to help proteins fold properly. Nucleic Acids Nucleic acids are used for: maintaining genetic continuity delivering information for protein synthesis energy molecule (ATP – adenosine triphosphate) Two major nucleic acid polymers: 1. 2. DNA – deoxyribonucleic acid RNA – ribonucleic acid Nucleic Acids DNA RNA located in the nucleus mainly found in cytoplasm double-stranded, double helix structure single-stranded structure stable molecule Nucleotides The basic subunit of nucleic acids is a nucleotide. Three components: 1. 2. 3. phosphate pentose sugar nitrogenous base Pentose Sugar Nitrogenous Bases purines pyrimidines Nucleic Acid reaction between 1. 2. pentose sugar OH group of one nucleotide phosphate group of another nucleotide forms phosphodiester bond In Conclusion The building blocks (monomers) of macromolecules are amino acids, nucleotides, simple sugars, and fatty acids In Conclusion • Name the two main chemical reactions shown making and breaking organic molecules In Conclusion Carbohydrates are use for energy storage and as structural materials Lipids are used as energy storage and structural components Proteins are made of amino acids which form structures, enzymes, transport, movement, and are part of the immune system Nucleic acids are the basis of inheritance and reproduction In Conclusion The versatility of carbon makes possible the great diversity of organic molecules Variation at the molecular level lies at the foundation of all biological diversity Answers to post-presentation activity #1 – the activity could be use alone as a pre-assessment (what should be known from Grade 11 SBI3U) Macromolecule 67 Example(s) of subunits Main functions Examples of macromolecules carbohydrates sugars (such as glucose) and polymers of glucose energy storage sugars, starches, and glycogen lipids glycerol and three fatty acids or glycerol and two fatty acids energy storage and cell membranes fats, oils, and phospholipids proteins polymers of amino acids transport, blood clotting, support, immunity, catalysis, and muscle action hemoglobin, fibrin, collagen, antibodies, enzymes, actin, and myosin nucleic acids polymers of nucleotides transfer and expression of genetic information DNA and RNA Day 2 68 • In building large macromolecules carbon usually combines with other carbons … AND with one or more functional groups 69 Why study Functional Groups? • These are the building blocks for organic molecules (or macromolecules – large organic molecules) 70 Why study Functional Groups? the components of organic molecules most commonly involved in chemical reactions the number and arrangement of functional groups give each organic molecule unique properties Types of functional groups • 6 functional groups most important to chemistry of life: hydroxyl carbonyl carboxyl 72 amino sulfhydryl phosphate Functional groups • they affect reactivity (e.g., hydrophilic, increase solubility in water) 73 Hydroxyl organic compounds with OH = alcohols alcohols, carbohydrates, nucleic acids, some acids, and steroids highly polar (makes molecules more soluble) e.g., ethanol Carbonyl if C=O at end molecule = aldelhyde if C=O in middle of molecule = ketone react with molecules (H-R2) to form H-R2-C-OH a ketone and an aldehyde may be structural isomers with different properties, e.g., acetone and propanal 75 Carboxyl compounds with COOH = acids fatty acids, amino acids acidic – tends to lose a proton (COO-) involved in peptide bonds e.g., acetic acid - gives vinegar its sour taste 76 Amino compounds with NH2 = amines amino acids, nucleic acids NH2 acts as base - can pick up a proton (H+) from the surrounding solution (ionized) under cellular conditions glycine (has amine and carboxyl groups) – replace an H with an R group to get an amino acid 77 Sulfhydryl compounds with SH = thiols 2 sulfhydryl groups can interact to help stabilize protein structure (S-S, disulfide bonds) 78 Phosphate • compounds called organic phosphates • Acidic – up to 2 negative charges when H+ dissociates • Links nucleotides in nucleic acids • Energy-carrier group in ATP 79 Review Review Review Do the functional groups make that much difference? identical basic structure of male & female hormones attachment of different functional groups interact with different targets in the body 83 2003-2004 A Bit of Honey: Bee keeping was used in Crete over 4000 years ago to allow for the collection of honey, a highly prized food having great value in ancient civilizations Field bees gather the nectar, a sweet secretion in plant blossoms that contains fructose, glucose and sucrose 84 Some worker bees secrete beeswax - Hexacosanoic acid, C26H52O2, and triacontanol, C30H62O