Macromolecules
advertisement

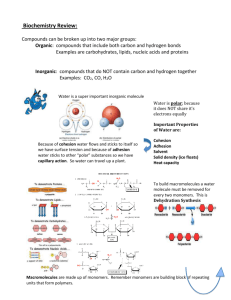
Macromolecules • All compounds on earth can be classified in 2 broad categories: – 1. Organic compounds- contains carbon and hydrogen atoms – 2. Inorganic compounds- can have one or the other, but do not contain BOTH carbon and hydrogen atoms Macromolecules • Most of your body’s molecules are ORGANIC! – Macromolecules are built from small organic compounds the same way a railroad train is built, by linking a lot of smaller unit together into long chains. Macromolecules • Large carbon compounds are built up from smaller simpler molecules called monomers (mono = ONE) Macromolecules • Monomers can bind to one another to form complex molecules known as polymers (poly = MANY) Macromolecules • A polymer consists of repeated, linked units, which can also bind forming large polymers called macromolecules (macro = LARGE) • Monomers link to form polymers through a chemical reaction called dehydration synthesis. During the formation of polymers, water is released or is a by-product. Dehydration Synthesis Macromolecules • The breakdown of some complex molecules, such as polymers, occurs through a process known as hydrolysis. This is the reverse of a condensation reaction or dehydration synthesis. The addition of water can break the bonds that hold polymers together. Hydrolysis Type of Macromolecules • • • • 1. Carbohydrates 2. Lipids 3. Proteins 4. Nucleic Acids Carbohydrates • Composed of carbon (C), hydrogen (H), and oxygen (O) atoms in the proportion of 1:2:1 – Example: The sugar glucose is a small carbohydrate; its chemical formula is C6H12O6 Carbohydrates • The building blocks, monomers, of carbs are called monosaccharides. Monosaccharides are simple sugars. – Examples: • Glucose- commonly found in the blood of animals • Galactose- a simple sugar found in milk • Fructose- commonly found in fruit Carbohydrates Carbohydrates • Disaccharides contain two monosaccharides joined during dehydration synthesis. – Examples: • Lactose- commonly found in milk and is made of galactose and glucose • Sucrose- commonly known as table sugar and is made of fructose and glucose Carbohydrates Carbohydrates • Polysaccharides are carbohydrates formed from linking of individual sugars into long chains. – Examples: • Starch- a common storage form of glucose in plants • Cellulose- found in the cell walls of plants; gives the plant strength and structure • Glycogen- a common storage form of glucose in animals (used for quick energy) Carbohydrates Lipids • These include fats, oils, waxes, and steroids • Do NOT dissolve in water (nonpolar) • Usually serve one of three functions: – 1. long term energy storage – 2. structural support in cell membranes (phospholipids) – 3. protection and insulation (especially in animals) Lipids • Fatty acids are the building blocks, or monomers, that make up most lipids. • Fatty acids can be saturated or unsaturated Proteins • Proteins are organic compounds mainly composed of carbon (C), hydrogen (H), and nitrogen (N) atoms. • Proteins are the construction materials for body parts such as hair, skin, nails, and blood. • Amino acids are the building blocks, monomers, that make up proteins. The individual amino acids are held together by peptide bonds. Proteins Proteins • One important group of proteins, enzymes, help control chemical reactions by acting as catalysts. Catalysts speed up reactions be lowering activation energy. Nucleic Acids • Nucleic acids are complex organic molecules that store genetic information in the cell. • Nucleotides are the building blocks, monomers, that make up most nucleic acids. Nucleic Acids • Nucleotides consist of a sugar, phosphate, and a nitrogen base – Example: DNA nucleotide Nucleic Acids • Three main types of nucleic acids: – 1. DNA: deoxyribonucleic acid; this is the genetic information inside the nucleus of all cells – 2. RNA: ribonucleic acid; instructions which code for proteins – 3. ATP: adenosine triphosphate; used as energy for the cell