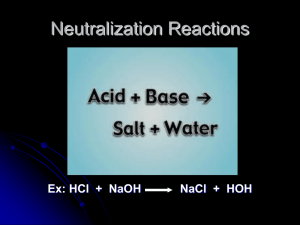
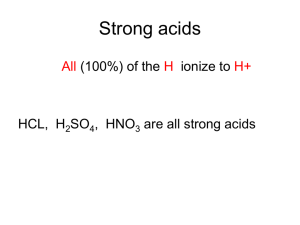
salt
advertisement
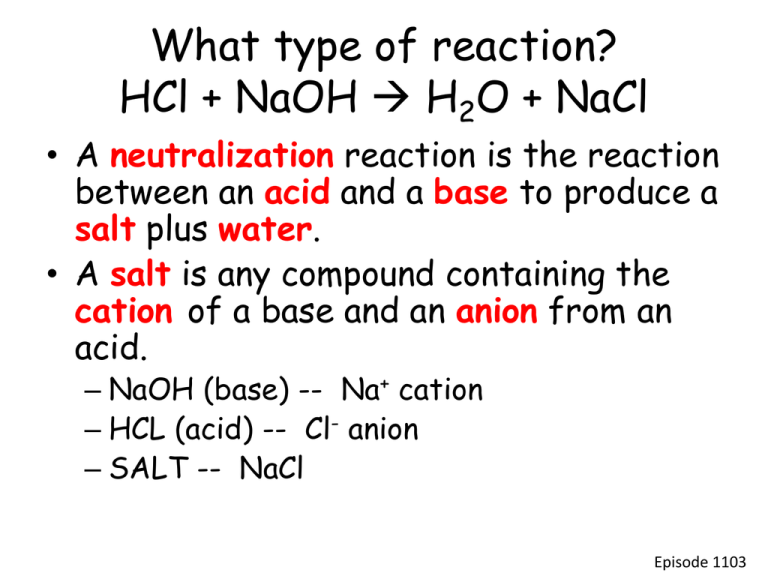
What type of reaction? HCl + NaOH H2O + NaCl • A neutralization reaction is the reaction between an acid and a base to produce a salt plus water. • A salt is any compound containing the cation of a base and an anion from an acid. – NaOH (base) -- Na+ cation – HCL (acid) -- Cl- anion – SALT -- NaCl Episode 1103 Note: Salt is not always NaCl. • Write the neutralization reaction when H2SO4 reacts with KOH. Label the acid, the base, and the salt. H2SO4 + 2 KOH 2 H2O + K2SO4 acid base salt Episode 1103 Write the neutralization reaction when nitric acid reacts with magnesium hydroxide. • Nitric acid – HNO3 • Magnesium hydroxide – Mg(OH)2 You know the products is going to be water and a salt • Formulate the salt • Write the equation and balance. 2HNO3 + Mg(OH)2 2H2O + Mg(NO3)2 Episode 1103 Titrations • A titration is a laboratory method used to determine the concentration of an acid or base in solution by performing a neutralization reaction with a standard solution. • In a neutral solution, the moles of hydrogen ions must be equal to the moles of hydroxide ions. Episode 1103 • To find moles of [H+] – Moles H+ = (#moles H+/1 moleacid )(Macid)(Vacid) • To find moles of [OH-] – Moles OH- = (#moles OH-/1 molebase )(Mbase)(Vbase) • If neutral, moles of [H+] = [OH-] – (#moles H+/1 moleacid )(Macid)(Vacid) = (#moles OH-/1 molebase )(Mbase)(Vbase) Episode 1103 Find the molarity of a sample of HCl by neutralizing it with 0.5 M NaOH. – Record volume of HCl solution. – Add indicator to HCl solution – Slowly add NaOH solution from burrette. • The endpoint of a titration is the point at which the indicator changes color indicating the neutralization has been reached so the moles of hydrogen ions and moles of hydroxide ions are equal. – Record volume of NaOH solution added. Episode 1103 Titration Calculation (#moles H+/1 molea )(Ma)(Va) = (#moles OH-/1 moleb )(Mb)(Vb) (1mol H+/1 mol HCl )(Ma)(50.0 mL) = (1 mol OH-/1 molNaOH )(0.5M)(20.2 ml) (Ma)(50.0 mL) = (0.5M)(20.2 ml) Ma = (0.5 M)(20.2 mL)/50.0 mL Ma= 0.20 M HCl Episode 1103 In a titration of HCl and KOH, 50.0 mL of the base were required to neutralize 10.0 mL of a 3.0 M HCl. What is the molarity of the KOH? (#moles H+/1 molea )(Ma)(Va) = (#moles OH-/1 moleb )(Mb)(Vb) (1mol H+/1 mol HCl )(3.0M)(10.0 mL) = (1 mol OH-/1 molNaOH )(Mb)(50.0 ml) (3.0M)(10.0 mL) = (Mb)(50.0 ml) Mb = (3.0M)(10.0 mL)/50.0 mL Mb= 0.60 M KOH Episode 1103 60.0 mL of 0.50 molar NaOH were needed to neutralize 30.0 mL of H2SO4. What is the molarity of the acid? (#moles H+/1 molea )(Ma)(Va) = (#moles OH-/1 moleb )(Mb)(Vb) (2mol H+/1 mol HCl )(Ma)(30.0 mL) = (1 mol OH-/1 molNaOH )(0.5M)(60.0 ml) 2(Ma)(30.0 mL) = (0.5M)(60.0 ml) Ma = (0.5 M)(60.0 mL)/(2)(30.0 mL) Ma= 0.50 M H2SO4 Episode 1103