Unit 1 - E Conservation of Atoms and Mass
advertisement

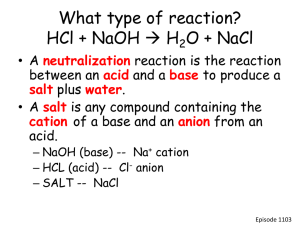
TOPIC E CONSERVATION OF ATOMS AND MASS • The law of conversation of mass is a fundamental part of chemistry. • You will need to relate this idea • as symbolic representations (equations), and • As particulate representations (drawings). • You might also need to perform calculations involving moles and masses when analyzing experimental data. • Solid carbon is known to react with oxygen to produce carbon dioxide. If a mass of 1.2 g of carbon is burned in oxygen, 4.4 g of carbon dioxide is found to form. How many grams of oxygen gas reacted with the carbon? • Which, if any, of the following representations of hydrogen gas • • • • • burning in oxygen gas to form water, accurately expresses the law of conservation of mass? In each case, explain your answer. (a) H2 + O2 H2O (b) H2 + 2O2 2H2O (c) 2H2 + O2 2H2O (d) H2 + H2 + O2 H2O + H2O More … • (e) • (f) Chemical equations, molar ratios and analysis of analytes • Use of moles is a key part of chemistry • if we know the number of moles of a substance and we have a balanced chemical equation • We can calculate the moles of another substance present in the equation. • Steps used: • 1. Write a correct, balanced equation. • 2. Find the number of moles present for one substance. • 3. Use the stoichiometric coefficients* (conversion factor) in the equation to find the number of moles of the unknown substance. • 4. Find the number of moles for the unknown substance. Gravimetric analysis and moles • Gravimetric analysis involves the addition of a substance to an aqueous solution to cause the formation of a solid. • The substance that is added is specifically chosen to react with the analyte (species undergoing analysis). • For example, addition of silver ions to a solution that contains chloride ions will result in the formation of a precipitate of silver chloride, according to the balanced equation below. • Cl-(aq) + Ag+(aq) AgCl(s) • When no more precipitate forms • the analyte (chloride ions) has been consumed, and • the stoichiometric molar ratio has been reached. • The solid is then separated from the solution by filtration and subsequent drying. • A solid of unknown composition contains some chloride ions. A 0.182 g sample of the solid is dissolved in water and the chloride ions dissolve to produce an aqueous solution. The solution has a large amount of aqueous silver ions added to it until no more solid can be formed. • After filtering and drying, it is found that 0.287 g of solid are produced in the reaction. • • (a) Identify the solid formed. • (b) Calculate the moles of the solid formed. • (c) Calculate the moles of chloride ions present in the original sample. • (d) Calculate the mass of chloride ions present in the original sample. • (e) Calculate the mass percentage of chloride ions in the original sample. • (f) What would the effect on the value calculated in (e) be, if the precipitate were only partially dried? Explain your answer. Titrations (volumetric analysis) and moles • Chemical reactions are often carried out between substances that are in solution. • The concentration of a solution can be measured in: • grams of the solute (solid) dissolved in a volume of solution. • Or number of moles of the solute in a particular volume of solution. • ( units = mol/L or mol L-1 called molarity (M)). M = mols / vol. (L) • And mols = M V • Example: • A solution has a concentration of 0.250 mol L-1 will have 0.250 moles of solute dissolved in 1.0 L of solution and can be referred to as ‘0.250 M solution’ or a ‘0.250 ‘molar’ solution’. Titration • Titration is the experimental method of analysis that uses concentrations of solutions. • If we know a balanced chemical equation we: • can calculate the moles of one substance, • then then determine the moles of other substances • And use that data to calculate an unknown concentration. • As in gravimetric analysis, we need to use a substance that specifically reacts with the analyte, • For example addition of a solution of hydrochloric acid to a solution of sodium hydroxide, will result in the formation of sodium chloride plus water according to the balanced equation below. • HCl(aq) + NaOH(aq) NaCl(aq) + H2O(l) • Since there is no solid formed, like in gravimetric analysis, we have to have another way of determining that the analyte has been totally consumed • We accomplish this by using an indicator that changes color at the equivalence point (the point at which the stoichiometric molar ratio has been achieved). • The observable event that occurs at the equivalence point is called the end point. • Hydroxides can be used to neutralize acids. • It is found that an indicator changes color at the precise moment that 44.0 mL of NaOH has been added to 25.0 mL of 2.00 mol L-1 HCI in a titration. • Use this date to calculate the concentration of NaOH.