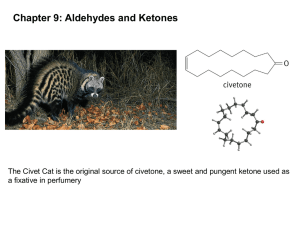
Reactions of Aldehydes and Ketones – Nucleophilic Addition
advertisement

REACTIONS OF ALDEHYDES AND KETONES – OXIDATION Aldehydes are oxidized to carboxylic acids by various oxidizing agents. Ketones oxidize with difficulty. They undergo slow cleavage with hot, alkaline KMnO4. 1 REACTIONS OF ALDEHYDES AND KETONES – NUCLEOPHILIC ADDITION REACTIONS B1. Addition of H– and R– Treatment of an aldehyde or ketone with either NaBH4 or LiAlH4 followed by protonation forms a 1° or 2° alcohol. 2 REACTIONS OF ALDEHYDES AND KETONES – NUCLEOPHILIC ADDITION REACTIONS B1. Addition of H– and R– 3 REACTIONS OF ALDEHYDES AND KETONES – NUCLEOPHILIC ADDITION REACTIONS B1. Addition of H– and R– Treatment of an aldehyde or ketone with either an organolithium (R”Li) or Grignard reagent (R”MgX) followed by water forms a 1°, 2°, or 3° alcohol. 4 REACTIONS OF ALDEHYDES AND KETONES – NUCLEOPHILIC ADDITION REACTIONS B1. Addition of H– and R– 5 REACTIONS OF ALDEHYDES AND KETONES – NUCLEOPHILIC ADDITION REACTIONS B2. Addition of CN– Aldehydes and unhindered ketones react with HCN to yield cyanohydrins. Because hydrogen cyanide is a toxic gas, best to use HCl and excess sodium cyanide. Addition of HCN is reversible and base-catalyzed. 6 REACTIONS OF ALDEHYDES AND KETONES – NUCLEOPHILIC ADDITION REACTIONS B2. Addition of CN– Cyanohydrins are important intermediates: 7 REACTIONS OF ALDEHYDES AND KETONES – NUCLEOPHILIC ADDITION REACTIONS B3. Reactions with Nitrogen Nucleophiles Aldehydes and ketones react with a primary amine to form an imine. An imine is a compound with a carbon–nitrogen double bond. Aldehydes and ketones react with a secondary amine to form an enamine. Enamines have a nitrogen atom bonded to a carbon–carbon double bond. REACTIONS OF ALDEHYDES AND KETONES – NUCLEOPHILIC ADDITION REACTIONS B4. Addition of alcohols The product formed when one equivalent of an alcohol adds to an aldehyde (ketone) is called a hemiacetal (hemiketal). The product formed when a second equivalent of alcohol is added to an aldehyde (ketone) is called an acetal (ketal). REACTIONS OF ALDEHYDES AND KETONES – NUCLEOPHILIC ADDITION REACTIONS B4. Addition of alcohols An alcohol is a poor nucleophile, so an acid catalyst is required for the reaction to take place at a reasonable rate. REACTIONS OF ALDEHYDES AND KETONES – NUCLEOPHILIC ADDITION REACTIONS B4. Addition of alcohols Acetals or ketals (being ethers) are chemically resistant to action of bases, oxidizing and reducing agents. However, they can be hydrolyzed back to aldehyde or ketone in acidic media. Ketals are hard to isolate except when in a cyclic form. REACTIONS OF ALDEHYDES AND KETONES – NUCLEOPHILIC ADDITION REACTIONS B4. Addition of alcohols Acetals are useful protective groups REACTIONS OF ALDEHYDES AND KETONES – NUCLEOPHILIC ADDITION REACTIONS B5. The Wittig Reaction The nucleophilic addition of phosphorus ylide to an aldehyde or a ketone to form an alkene is called a Wittig reaction. The sequence converts C=O is to C=C. REACTIONS OF ALDEHYDES AND KETONES – NUCLEOPHILIC ADDITION REACTIONS B5. The Wittig Reaction The phosphorus-containing reagent is called a phosphorane, and it belongs to a larger class of compounds called ylides. An ylide is a compound with two oppositely charged atoms adjacent to each other. REACTIONS OF ALDEHYDES AND KETONES – NUCLEOPHILIC ADDITION REACTIONS B5. The Wittig Reaction Wittig reagents are easily prepared by treating triphenylphosphine with an alkyl halide followed by a strong base: (BuLi, NaH, NaNH2). REACTIONS OF ALDEHYDES AND KETONES – NUCLEOPHILIC ADDITION REACTIONS B5. The Wittig Reaction Advantages of the Wittig reaction over other methods of preparing alkenes: position of double bond is always clear, no rearrangement of carbon skeleton. Complete the reactions. If no reaction occurs write N.R. REACTIONS OF ALDEHYDES AND KETONES – REACTIONS AT THE ALPHA CARBON C1. Acidity of the α Hydrogen The α proton is quite acidic (pKa = 16-20) The β proton is not acidic (pKa = 40-50) 22 REACTIONS OF ALDEHYDES AND KETONES – REACTIONS AT THE ALPHA CARBON C1. Acidity of the α Hydrogen The acidity of alpha proton is rationalized by considering resonance stabilization of conjugate base, the enolate an ion. 23 REACTIONS OF ALDEHYDES AND KETONES – REACTIONS AT THE ALPHA CARBON C1. Acidity of the α Hydrogen The acidity of an α hydrogen, between two carbonyl groups, is even greater (pKa ≈ 9) 24 REACTIONS OF ALDEHYDES AND KETONES – REACTIONS AT THE ALPHA CARBON C2. Keto-Enol Tautomerism The negative charge of the enolate anion is distributed on both oxygen and carbon, the ion can combine with a proton at either site. 25 REACTIONS OF ALDEHYDES AND KETONES – REACTIONS AT THE ALPHA CARBON C2. Keto-Enol Tautomerism The negative charge of the enolate anion is distributed on both oxygen and carbon, the ion can combine with a proton at either site. They are constitutional isomers = tautomers. They interconvert rapidly in the presence of catalytic amounts of acids or bases = tautomerization. The keto form, generally, is heavily favored in the equilibrium. 26 REACTIONS OF ALDEHYDES AND KETONES – REACTIONS AT THE ALPHA CARBON C2. Keto-Enol Tautomerism In β-dicarbonyls, the amount of enol tautomer present at equilibrium is much higher due to resonance of conjugated system and intramolecular hydrogen bonding. 27 How many acidic hydrogens does each of the molecules have? Draw structures for the enol tautomers. (a) cyclopentanone 4 (b) acetyl chloride 3 (c) ethyl acetate 3 (d) propanal 2 (e) acetic acid 4 REACTIONS OF ALDEHYDES AND KETONES – REACTIONS AT THE ALPHA CARBON C3. Alpha-Halogenation Aldehydes and ketones with at least one α-hydrogen react at the α-carbon with bromine, chlorine, or iodine. The reaction is catalyzed by both acid and base. REACTIONS OF ALDEHYDES AND KETONES – REACTIONS AT THE ALPHA CARBON C3. Alpha-Halogenation Mechanism of bromination of acetone in acetic acid: REACTIONS OF ALDEHYDES AND KETONES – REACTIONS AT THE ALPHA CARBON C3. Alpha-Halogenation Mechanism of bromination of acetone in acetic acid: REACTIONS OF ALDEHYDES AND KETONES – REACTIONS AT THE ALPHA CARBON C3. Alpha-Halogenation Mechanism of bromination of acetone in acetic acid: REACTIONS OF ALDEHYDES AND KETONES – REACTIONS AT THE ALPHA CARBON C4. The Aldol Addition Two molecules of an aldehyde or ketone (with α-hydrogens) react with each other in the presence of a base to form a -hydroxy aldehyde or ketone. REACTIONS OF ALDEHYDES AND KETONES – REACTIONS AT THE ALPHA CARBON C4. The Aldol Addition Mechanism for the aldol addition: REACTIONS OF ALDEHYDES AND KETONES – REACTIONS AT THE ALPHA CARBON C4. The Aldol Addition Ketones are less susceptible than aldehydes to attack by nucleophiles, so aldol additions occur more slowly with ketones. With ketones, the reaction proceeds well only if the product is removed from the basic solution or reacts further by dehydration. REACTIONS OF ALDEHYDES AND KETONES – REACTIONS AT THE ALPHA CARBON C4. The Aldol Addition When heated in acidic or basic conditions, the product of an aldol addition reaction will undergo elimination to produce unsaturation between the α and β positions: α,β-unsaturated aldehyde or ketone. REACTIONS OF ALDEHYDES AND KETONES – REACTIONS AT THE ALPHA CARBON C4. The Aldol Addition Mechanism for dehydration: REACTIONS OF ALDEHYDES AND KETONES – REACTIONS AT THE ALPHA CARBON C4. The Aldol Addition The two-step process (aldol addition plus dehydration) is called an aldol condensation. It is possible to carry out an aldol reaction between two different carbonyl compounds. Such reactions are called crossed or mixed aldol reactions. It requires that only one of the reactants is capable of forming an enolate (possesses an α-H). REACTIONS OF ALDEHYDES AND KETONES – REACTIONS AT THE ALPHA CARBON C4. The Aldol Addition The products are very susceptible to dehydration when heated. 40 41 SPECTROSCOPY A. Mass Spectrometry Cleavage of the bond between the carbonyl group and the carbon yields a neutral radical and an oxygen-containing cation. SPECTROSCOPY B. Infrared Spectroscopy Aldehydes and ketones show a strong C=O peak at 1660 to 1770 cm–1 Aldehydes show two characteristic C–H absorptions in the 2720 to 2820 cm–1 range. SPECTROSCOPY C. Ultra-Violet Spectroscopy UV-Vis active but not very useful SPECTROSCOPY D. 1H NMR Aldehyde proton signals are at δ 10 Protons on the carbon to the carbonyl group absorb at δ 2.0 to δ 2.5 SPECTROSCOPY E. 13C NMR C=O signal is at δ190 to δ215. No other kinds of carbons absorb in this range. 47