CHM-373 American Women in Science and Society

Chapter 18:
Ketones and Aldehydes
Classes of Carbonyl Compounds
Carbonyl
• C=O bond is shorter, stronger and more polar than C=C bond in alkenes
Nomenclature: Ketone
• Number chain so the carbonyl carbon has the lowest number
• Replace “e” with “one”
Nomenclature: Cyclic Ketone
• Carbonyl carbon is #1
Nomenclature: Aldehydes
• Carbonyl carbon is #1
• Replace “e” with “al”
• If aldehyde is attached to ring, suffix
“carbaldehyde” is used
Nomenclature
• With higher-priority functional groups, ketone is
“oxo” and an aldehyde is a “formyl” group
• Aldehydes have higher priority than ketones
Nomenclature- Common Names: Ketones
• Name alkyl groups attached to carbonyl
• Use lower case Greek letters instead of numbers
Nomenclature
Boiling Points
• Ketones and aldehydes are more polar. Have higher boiling point that comparable alkanes or ethers
Solubility: Ketones and Aldehydes
• Good solvent for alcohols
• Acetone and acetaldehyde are miscible in water
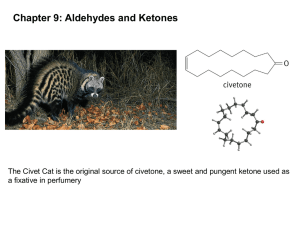
Formaldehyde
• Gas at room temperature
IR Spectroscopy
• Strong C=O stretch around 1710 cm -1 (ketones) or 1725 cm -1 (simple aldehydes)
• C-H stretches for aldehydes: 2710 and 2810 cm -1
IR Spectroscopy
• Conjugation lowers carbonyl frequencies to about 1685 cm -1
• Rings with ring strain have higher C=O frequencies
Proton NMR Spectra
• Aldehyde protons normally around δ9-10
• Alpha carbon around δ2.1-2.4
Carbon NMR Spectra
Mass Spectrometry (MS)
Mass Spectrometry (MS)
Mass Spectrometry (MS)
McLafferty Rearrangement
• Net result: breaking of the
, bond and transfer of a proton from the
carbon to oxygen
Ultraviolet Spectra of Conjugated Carbonyls
• Have characteristic absorption in UV spectrum
• Additional conjugate C=C increases
max about
30 nm, additional alkyl groups increase about
10nm
Carbonyl Electronic Transitions
Industrial Uses
• Acetone and methyl ethyl ketone are common solvents
• Formaldehyde is used in polymers like Bakelite and other polymeric products
• Used as flavorings and additives for food
Industrial Uses
Synthesis of Aldehydes and Ketones
• The alcohol product of a Grignard reaction can be oxidized to a carbonyl
Synthesis of Aldehydes and Ketones
• Pyridinium chlorochromate (PCC) or a Swern oxidation takes primary alcohols to aldehydes
Synthesis of Aldehydes and Ketones
• Alkenes can be oxidatively cleaved by ozone, followed by reduction
Synthesis of Aldehydes and Ketones
• Friedel-Crafts Acylation
Synthesis of Aldehydes and Ketones
• Hydration of Alkynes
• Involves a keto-enol tautomerization
• Mixture of ketones seen with internal alkynes
Synthesis of Aldehydes and Ketones
• Hydroboration-oxidation of alkyne
• Anti-Markovnikov addition
Synthesis Problem
Synthesis of Aldehydes and Ketones
• Organolithium + carboxylic acid ketone (after dehydration)
Synthesis of Aldehydes and Ketones
• Grignard or organolithium reagent + nitrile ketone (after hydrolysis)
Synthesis of Aldehydes and Ketones
• Reduction of nitriles with aluminum hydrides will afford aldehydes
Synthesis of Aldehydes and Ketones
• Mild reducing agent lithium aluminum tri(tbutoxy)hydride with acid chlorides
Synthesis of Aldehydes and Ketones
• Organocuprate (Gilman reagent) + acid chloride ketone
Nucleophilic Addition
• Aldehydes are more reactive than ketones
Wittig Reaction
• Converts the carbonyl group into a new C=C bond
• Phosphorus ylide is used as the nucleophile
Wittig Reaction
• Phosphorus ylides are prepared from triphenylphosphine and an unhindered alkyl halide
• Butyllithium then abstracts a hydrogen from the carbon attached to phosphorus
Wittig Reaction- Mechanism
• Betaine formation
• Oxaphosphetane formation
Wittig Reaction- Mechanism
• Oxaphosphetane collapse
How would you synthesize the following molecule using a Wittig Reaction
Hydration of Ketones and Aldehydes
• In aqueous solution, a ketone or aldehyde is in equilibrium with it’s hydrate
• Ketones: equilibrium favors keto form
Hydration of Ketones and Aldehydes
• Acid-Catalyzed
Hydration of Ketones and Aldehydes
• Base-Catalyzed
Cyanohydrin Formation
• Base-catalyzed nucleophilic addition
• HCN is highly toxic
Formation of Imines
• Imines are nitrogen analogues of ketones and aldehydes
• Optimum pH is around 4.5
Formation of Imines- Mechanism
Condensations with Amines
Acetal Formation
Hemiacetal Formation- Mechanism
• Must be acid-catalyzed
Acetal Formation- Mechanism
• Must be acid-catalyzed
Hydrolysis of Acetals
• Acetals can be hydrolyzed by addition of dilute acid
• Excess of water drives equilibrium towards carbonyl formation
Cyclic Acetals
• Addition of diol produces cyclic acetal
• Reaction is reversible
• Used as a protecting group
• Stable in base, hydrolyze in acid
Cyclic Acetals- Protecting Group
O
O
O
H
1) NaBH
4
2) H
3
O
+
OH
O
H
• Acetals are stable in base, only ketone reduces
• Hydrolysis conditions protonate the alkoxide and restore the aldehyde
Oxidation of Aldehydes
• Easily oxidized to carboxylic acids
Tollens Test
• Involves a solution of silver-ammonia complex to the unknown compound
• If an aldehyde is present, its oxidation reduces silver ion to metallic silver
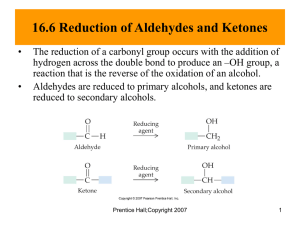
Reducing Reagents- Sodium Borohydride
• NaBH
4 can reduce ketones and aldehydes, not esters, carboxylic acids, acyl chlorides, or amides
Reducing Reagents- Lithium Aluminum Hydride
• LiAlH
4 can reduce any carbonyl
O
R R(H) aldehyde or ketone
LiAlH
4 ether
OH
R
H
R(H)
Reducing Reagents- Catalytic Hydrogenation
• Widely used in industry
• Raney nickel is finely divided Ni powder saturated with hydrogen gas
• Will attack alkene first, then carbonyl
Deoxygenation of Ketones and Aldehydes
• Clemmensen reduction or Wolff-Kishner reactions can deoxygenate ketones and aldehydes
Clemmensen Reduction
• Uses Zinc-Mercury amalgam in aqueous HCl
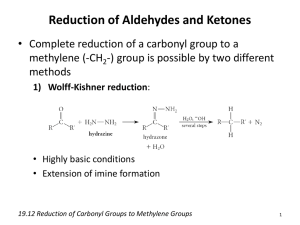
Wolff-Kishner Reduction
• Forms hydrazone, then needs heat with strong base like KOH or potassium tert-butoxide
• Use high-boiling solvent (ethylene glycol, diethylene glycol, or DMSO)