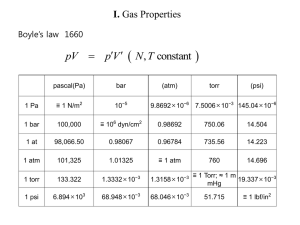
Document
advertisement

refers to the use of external techniques to probe into the internal structure and properties of a material. GEL PERMEATION CHROMATOGRAPHY DIFFERENTIAL SCANNING CALORIMETRY THERMOGRAVEMETRIC ANALYSIS INFRA RED SPECTROSCOPY POROSITY TECHNIQUES FOR PROSITY ANALYSIS Mikhail Tswett, Russian, 1872-1919 Botanist In 1906 Tswett used to chromatography to separate plant pigments He called the new technique chromatography because the result of the analysis was 'written in color' along the length of the adsorbent column Chroma means “color” and graphin means to “write” …is a technique used to separate and identify the components of a mixture. Works by allowing the molecules present in the mixture to distribute themselves between a stationary and a mobile medium. Mobile phase Mobile phase is a liquid as water or dilute alcohol Separation mechanism Based on difference between the solutes molecular weights. Molecules will distribute themselves outside & inside the pores according to their size. This type is also known as: GPC can determine several important parameters. These include number average molecular weight, weight average molecular weight, and the most fundamental characteristic of a polymer its molecular weight distribution. These values are important, since they affect many of the characteristic physical properties of a polymer. Tensile strength Elastomer relaxation time Brittleness Impact strength Toughness Adhesive strength Cure time Elastic modulus Hardness Softening temperature "Size Exclusion" chromatography Molecular weight distribution Polymer solution small molecules Column medium molecules Large molecules Number average Molecular weight Separation based on Size Weight average Molecular weight Size exclusion chromatography Large particles cannot enter gel and are excluded. They have less volume to traverse and elute Small particles can enter gel and have more volume to traverse. They elute later chromatogram flow time How GPC works SEC was first developed in 1955 by Lathe and Ruthven GPC separates molecules in solution by their "effective size in solution." To prepare a sample for GPC analysis the polymer sample is first dissolved in an appropriate solvent. Inside the gel permeation chromatograph, the dissolved sample is injected into a continually flowing stream of solvent (mobile phase). The mobile phase flows through millions of highly porous, rigid particles (stationary phase) tightly packed together in a column. The pore sizes of these particles are controlled and available in a range of sizes. Schematic of a basic Gel permeation chromatograph sample Data system Solvent delivery system Solvent supply Mobile phase injector Column(s) detector(s) Small molecules penetrate pores of particles Large molecules are excluded Polymer – Dissolving in solvent Membranes – Passed through std. sol. MWCO (Molecular weight Cut-Off) High temperature GPC Basic Principles of Thermal Analysis Modern instrumentation used for thermal analysis usually consists of the following parts: sample holder/compartment for the sample sensors to detect/measure a property of the sample and the temperature an enclosure within which the experimental parameters (temperature, speed, environment) may be controlled a computer to control data collection and processing Temperature Control (Furnace) sample Sensors PC Differential two calorimeters (for sample and reference) with the same heat transfer behavior (for compensation purpose) Scanning the common operation mode is to run temperature or time scans Calorimeter instrument to measure heat or heat flow Amorphous Phase Crystalline Phase Semi-crystalline Polymers Melting The portion of material whose molecules are randomly oriented in space. Liquids and glassy or rubbery solids. Thermosets and some thermoplastics. The portion of material whose molecules are regularly arranged into well defined structures consisting of repeat units. Very few polymers are 100% crystalline. Polymers whose solid phases are partially amorphous and partially crystalline. Most common thermoplastics are semi-crystalline. The endothermic transition upon heating from a crystalline solid to the liquid state. This process is also called fusion. The melt is another term for the polymer liquid phase. Semi-Crystalline (or Amorphous) Crystalline Phase melting temperature Tm (endothermic peak) Amorphous Phase glass transition temperature (Tg) T g < Tm Crystallisable polymer can crystallize on cooling from the melt at Tc (Tg < Tc < Tm) Gas control Furnace differential detector temperature controller signal amplifier furnace Sample Reference Temperature controller Detectors gas control device data acquisition device Microvolt amplifier Data acquisition Sample Pan Pan sealed DSC measures the temperatures and heat flows associated with transitions in materials as a function of time and temperature in a controlled atmosphere. These measurements provide quantitative and qualitative information about physical and chemical changes that involve endothermic or exothermic processes, or changes in heat capacity. Heat Flow - > exothermic Typical DSC Curve of a Thermosetting Polymer Cross -Linking (Cure) Crystallization Glass Transition Melting Temperature Oxidation Typical Features of a DSC Trace Exothermic upwards Endothermic downwards Melting (Tm) (Tc) Glass Transition (TCrystallization g) Desolvation H 2O Decomposition Y-axis – heat flow X-axis – temperature (and time) Temperature (oC) DSC Curve : Heat/Cool/Heat 1.5 cooling Heat Flow (W/g) 1.0 0.5 first heating 0.0 -0.5 second heating -1.0 -1.5 0 40 80 120 Temperature (°C) 160 200 240 Onset = Melting point (mp) ^exo 20 mW Heat of fusion (melting) = integration of peak 40 60 80 100 120 140 160 180 200 220 240 260 280 300 o temperature(o[C) C] Temperature Influence of Sample Mass DSC Heat Flow (W/g) 0 Indium at 10°C/minute Normalized Data -2 15mg Onset not influenced by mass 10mg 4.0mg -4 1.7mg 1.0mg 0.6mg -6 150 152 154 156 158 160 Temperature (°C) 162 164 166 A technique measuring the variation in mass of a sample undergoing temperature scanning in a controlled atmosphere Thermobalance allows for monitoring sample weight as a function of temperature The sample hangs from the balance inside the furnace and the balance is thermally isolated from the furnace Gas IN Sample: RCD 1 Size: 4.9250 mg Method: Ramp TGA File: D:\TGA-DSC\TGA\NafionRCD_1.008 Operator: LK Run Date: 10-Sep-2012 17:52 Instrument: TGA Q500 V20.13 Build 39 120 100 Weight (%) 80 60 40 20 0 0 200 400 Temperature (°C) 600 800 Universal V4.5A TA Instruments TGA in Nitrogen and Oxygen atmosphere % % 100 Nitrogen IDT 80 Weight Loss Weight Loss 80 Oxygen 60 40 Poly(PMDA-ODA-IPC) 20 200 300 Poly(PMDA-ODA-IPC Poly(PMDA-ODA-TPC) Poly(PMDA-ODA-TMAc) 100 40 20 Poly(PMDA-ODA-TPC) 0 60 400 500 Temperature 600 700 800 °C CD Poly(PMDA-ODA-TMAc) 0 100 200 300 400 500 Temperature Data : IDT - Initial decomposition Temp Weight Vs Time CD - Complete decomposition Weight Vs Temperature 600 700 800 °C Weight Loss (%) Step-I Step-II Step-III Temperature (oC) The entire electromagnetic spectrum is used by chemists: ~1019 ~1017 ~1015 ~1013 ~1010 ~105 ~.0001 nm ~0.01 nm 10 nm 1000 nm 0.01 cm 100 m ~10-4 ~10-6 > 300 g-rays X-rays nuclear core excitation (PET) electron excitation (X-ray cryst.) 300-30 300-30 UV electronic excitation (p to p*) IR molecular vibration Visible Microwave molecular rotation Radio Nuclear Magnetic Resonance NMR A molecule such as H2O will absorb infrared light when the vibration (stretch or bend) results in a molecular dipole moment change Symmetric Stretch Asymmetric Stretch Bend A molecule can be characterized (identified) by its molecular vibrations, based on the absorption and intensity of specific infrared wavelengths. Water O-H Bend O-H Stretching The Infrared region is divided into: near, mid and far-infrared. – Near-infrared refers to the part of the infrared spectrum that is closest to visible light and farinfrared refers to the part that is closer to the microwave region. – Mid-infrared is the region between these two The four primary regions of the IR spectrum Bonds to H Triple bonds OH single bond NH single bond CH single bond Single Bonds Double bonds CC CN CO C=O C=N C=C C≡C C≡N Fingerprint Region 4000 cm-1 2700 cm-1 2000 cm-1 1600 cm-1 400 cm-1 Non-porous solid Low specific surface area Low specific pore volume Porous solid High specific surface area High specific pore volume Porous materials have highly developed internal surface area that can be used to perform specific function. Almost all solids are porous except for ceramics fired at extremely high temperatures Inter-connected (open) closed Open pores are accessible whereas closed pores are inaccessible pores. Open pores can be inter-connected, passing or dead end. Passing (open) Dead end (open) Size of Pores (IUPAC Standard) Macropores Mesopores Micropores Zeolite, Activated carbon, Metal organic framework Mesoporous silica, Activated carbon 2 nm Sintered metals and ceramics 50 nm Porous material are classified according to the size of pores: material with pores less than 2 nm are called micropores, materials with pores between 2 and 50 nm are called mesopores, and material with pores greater than 50 nm are macrospores Gas adsorption Gas adsorption Small angle Neutron scattering Mercury Porosimetry Techniques Small angle X-ray scattering TEM SEM Typical Pore Structure Conclusion Gel permeation chromatography (GPC) is a type of chromatography (SEC), that separates analytes on the basis of size. size exclusion DSC is a thermoanalytical technique in which the difference in the amount of heat required to increase the temperature of a sample and reference is measured as a function of temperature TGA is a type of testing performed on samples that determines changes in weight in relation to a temperature program in a controlled atmosphere. IR spectroscopy used to identify and study chemical structure of material in the Infra red region Porous material : micropores, mesopores and macropores