Carboxylic Acids
advertisement

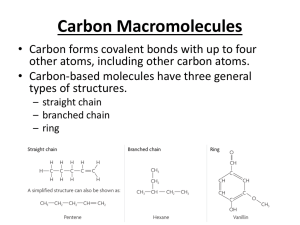
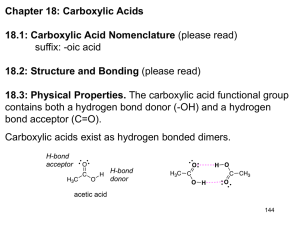
Carboxylic Acids The functional group of a carboxylic acid is a carboxyl group, which can be represented in any one of three ways. Nomenclature IUPAC names: • For an acyclic carboxylic acid, take the longest carbon chain that contains the carboxyl group as the parent alkane. • Drop the final -e from the name of the parent alkane and replace it by -oic acid. • Number the chain beginning with the carbon of the carboxyl group. • Because the carboxyl carbon is understood to be carbon 1, there is no need to give it a number. Nomenclature • In these examples, the common name is given in parentheses. • An -OH substituent is indicated by the prefix hydroxy-; an -NH2 substituent by the prefix amino-. Nomenclature To name a dicarboxylic acid, add the suffix -dioic acid Nomenclature Nomenclature For common names, use, the Greek letters alpha (a), beta (b), gamma (g), and so forth to locate substituents. Physical Properties The carboxyl group contains three polar covalent bonds; C=O, C-O, and O-H. The polarity of these bonds determines the major physical properties of carboxylic acids. Physical Properties The higher boiling points are a result of their polarity and the fact that hydrogen bonding between two carboxyl groups creates a dimer that behaves as a highermolecular-weight compound. Physical Properties Carboxylic acids are more soluble in water than are alcohols, ethers, aldehydes, and ketones of comparable molecular weight. Fatty Acids Fatty acids: Long chain carboxylic acids derived from animal fats, vegetable oils, or phospholipids of biological membranes. • More than 500 have been isolated from various cells and tissues. • Most have between 12 and 20 carbons in an unbranched chain. • In most unsaturated fatty acids, the cis isomer predominates; trans isomers are rare. Fatty Acids The Most Abundant Fatty Acids in Animal Fats, Vegetable Oils, and Biological Membranes. Fatty Acids Saturated fatty acids are solids at room temperature. • They pack close together to maximize interactions (by London dispersion forces) between their chains. Fatty Acids All unsaturated fatty acids are liquids at room temperature because the cis double bonds interrupt the regular packing of their hydrocarbon chains. Fatty Acids Unsaturated fatty acids can have more than one double bond which is almost always cis Soaps • Soaps are sodium salts or potassium salts of fatty acids. • They are prepared from triglycerides. • Triglycerides are triesters of glycerol. • The reaction that takes place is called saponification (Latin: saponem, “soap”). Soaps In water, soap molecules cluster into micelles. The hydrophobic tails are shielded from the aqueous environment, and their hydrophilic heads are in contact with the aqueous environment. Soaps When soap and dirt are mixed in water, the nonpolar hydrocarbon tails “dissolve” the nonpolar substances such as grease and oil. Soaps • Soaps form water-insoluble salts in hard water. • Hard water contains Ca2+, Mg2+, and Fe3+ ions. Detergents The problem of formation of precipitates in hard water was overcome by using a molecule containing a sulfonate (-SO3- ) group in the place of a carboxylate (-CO2-) group. • Calcium, magnesium and iron salts of sulfonic acids, RSO3H, are more soluble in water than are their salts of fatty acids. Acidity of Carboxylic Acids • Carboxylic acids are weak acids. • Values of Ka for most unsubstituted aliphatic and aromatic carboxylic acids fall within the range 10-4 to 10-5 (pKa 4.0 - 5.0). Acidity of Carboxylic Acids When a carboxylic acid is dissolved in aqueous solution, the form of the carboxylic acid present depends on the pH of the solution in which it is dissolved. Reaction with Bases Carboxylic acids react with NaOH, KOH, and other strong bases to form water-soluble salts. They also form water-soluble salts with ammonia and amines. Fischer Esterification In Fischer esterification, a carboxylic acid is reacted with an alcohol in the presence of an acid catalyst, most commonly concentrated sulfuric acid. Fischer esterification is reversible. It is possible to drive it in either direction by the choice of experimental conditions (Le Chatelier’s principle). Fischer Esterification • In Fischer esterification, the alcohol adds to the carbonyl group of the carboxylic acid to form a tetrahedral carbonyl addition intermediate. • The intermediate then loses H2O to give an ester. Decarboxylation • Decarboxylation: The loss of CO2 from a carboxyl group. • Many carboxylic acids, when heated to a very high temperature, will undergo thermal decarboxylation. • Most carboxylic acids, however, are resistant to moderate heat and melt and even boil without undergoing decarboxylation. • An exception is any carboxylic acid that has a carbonyl group on the carbon b to the COOH group. Decarboxylation • Decarboxylation of a b-ketoacid. • The mechanism forms a enol tautomer. Decarboxylation • An important example of decarboxylation of a b-ketoacid in biochemistry occurs in the Krebs cycle. • Oxalosuccinic acid, one of the intermediates in this cycle, has a ketone b to one of the three carboxyl groups. Structure Summary • Polar Groups • Monoacids form dimers through hydrogen bonding • Diacids have two carboxyl groups • Long-chain acids are called fatty acids • Fatty acids are saturated or unsaturated • Double bonds of fatty acids are cis • Fatty Acid salts are called soaps Reactions Summary • Acid Base Reactions • Reduction with LAH • Fischer Esterification • Decarboxylation • Esters of Sulphonic Acids and Phosphoric Acid • Anhydrides of Carboxylic Acids and Phosphoric Acid Reactions Summary Acid Base Reactions • Reaction with bases give carboxylates (salts) O H 3C O ONa O NaO H H 3C OH KOH H 3C • Reaction with ammonium give ammonium salts O O H N H 3 O O N H 4 OK Reactions Summary • Reduction with LAH O OH LAH H 3C OH H 3O + H 3C OH Reactions Summary • Esterification Reactions Summary • Decarboxylation Related Chemistry of Acids • Sulfonic Acid and Phosphoric Acid also form Esters O SO H O O C H O H 3 + H O 3 O H O PO H O C H O H 3 + H O 3 SO C H O 3 +H 2 O O PO C H O H O 3 +H 2 O Related Chemistry of Acids • Carboxylic Acids Condense to form Anhydrides O O O O + + H 3 C O H H O C H 3 H 3 C C H O H 2 O 3 • Phosphoric Acid also forms Anhydrides O O H O PO H+ H O PO H O O OO O POPO H H O O O P h o s p h o r ic A c id A n h y d r id e a ls o c a lle d a d ip h o s p h a t e O P P O H H OO + PH H OO OO O O O OO P P O P O H H OO O OO t r i p h o s p h a t e