YS_saxitoxin
advertisement
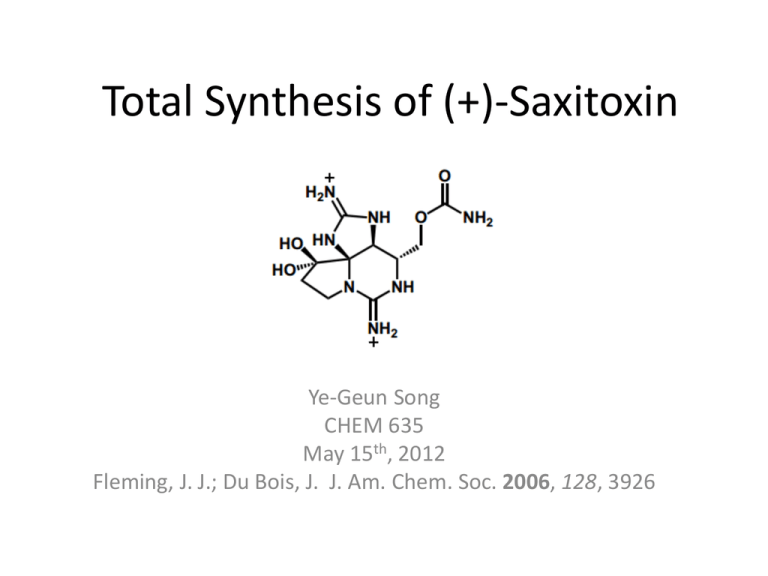
Total Synthesis of (+)-Saxitoxin Ye-Geun Song CHEM 635 May 15th, 2012 Fleming, J. J.; Du Bois, J. J. Am. Chem. Soc. 2006, 128, 3926 Saxitoxin • A neurotoxin naturally produced by certain species of marine dinoflagellates (plankton) and cyanobacteria (green algae). • Is responsible for the paralytic shellfish poisoning (PSP) in human. No cure known. • Found in at least 12 marine puffer fish species in Asia and Brazil, but the ultimate source unknown. In the USA, the PSP is limited to New England and the West Coast. • Acts as a selective sodium channel blocker. It acts on the voltage-gated Na+ channels of nerve cells, preventing normal cellular of nerve cells, preventing normal cellular function and leading to paralysis (Medicinal interest) • Toxicity of 8µg/Kg in mice; 0.2-1.0 mg would prove fatal to humans;100x more poisonous than strychnine, 1000x (sarin gas), 2000x (NaCN) (Millitary interest) The Challenge for Chemical Synthesis • Dense arrangement of heteroatoms • 3 contiguous stereocenters • Tricyclic skeleton possessing two guanidine groups • The dicationic nature of the molecule complicates the manipulation/purification Total Syntheses of Saxtoxin • 1977 – First by Kishi; racemic saxitoxin • 1984 – Second by Jacobi; racemic saxitoxin • 2006 – Third by Du Bois; natural (+)-saxitoxin using commercially available SM, (R)-glycerol acetamide. (JACS. 2006, 128, 3926) Retrosynthesis Forward Synthesis: Oxathiazinane 6 Step 3 -> 4: oxathiazinane iminium ion equivalent Proposed TS: Nucleophilic additions to tetrahydropyridinium ions Axial attack by the alkynyl anion on the twist-chair form of the iminium intermediate would give the cis-C4,C5 stereochemistry. Not confirmed yet. Du Bois, J. et. al, J. Am. Chem. Soc. 2003, 125, 2028 Forward Synthesis: Azide 8 Forward Synthesis: 9-membered ring 10 Forward Synthesis: (+)-STX β-saxitoxinol Conclusion • 1.6% overall yield • First total synthesis of (+) saxitoxin • Stereocontrolled formation of the 9-membered ring and condensation to prepare the bicyclic guanidine core • 19 steps from commercially available (R)- glycerol acetamide











