MOCVD Basics & Applications: Thin Film Deposition
advertisement
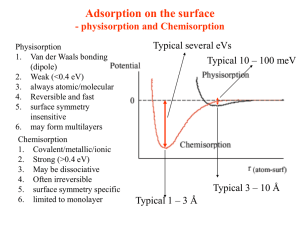
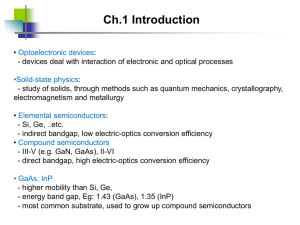
MOCVD Basics & Applications Sisay Outline Introduction Advantages/Disadvantages Basic transport and growth mechanisms Application What is MOCVD? MOCVD stands for Metal-Organic Chemical Vapour Deposition. MOCVD is a technique that used to grow/deposit thin solid films, usually semiconductors, on solid substrates (wafers)using organo metallic compounds as sources. The films grown by MOCVD are mainly used for the fabrication of electronic and optoelectronic devices. The electronic and optoelectronic devices produced by MOCVD are used in cell phones , optical communication, optical storage (CD, DVD), traffic lights, bill boards (LEDs), lighting and solar cells. Using MOCVD we can build up many layers, each of a precisely controlled thickness, to create a material which has specific optical and electrical properties. Overview of Epitaxy Techniques Technique Strengths Weaknesses LPE (liguid phase epitaxy) Simple, High purity Scale economies Inflexible, Non-uniformity HVPE ( hydride vapor phase epitaxy) Well developed Large scale No Al alloys Complex process/reactor control difficult, Hazardous sources MBE Simple process, Uniform, Abrupt interface In-situ monitoring As/P alloy difficult, Expensive , Low throughput MOCVD OMVPE OMCVD MOVPE Most flexible, Large scale production Abrupt interface Simple reactor, High purity, selective in situ monitoring Expensive sources Most parameters to control Accurately Hazardous precursors Why MOCVD? High grown layers quality Faster growth rate than MBE, can be a few microns per hour; multiwafer capability easily achievable Doping uniformity/reproducibility High throughput and no ultra high vacuum needed (compared to MBE), Economically advantageous. Highest flexibility, Different materials can be grown in the same system. Precision in deposition thickness and possible sharp interfaces growth –thus, it is very suitable for hetero-structures, e.g., multi quantum wells (MQW) Higher temperature growth; growth process is thermodynamically favorable Disadvantages Many materials that we wish to deposit have very low vapour pressures and thus are difficult to transport via gases Not abruptable process as MBE due to gas flow issues Human Hazard ,that is, Toxic and corrosive gases are to be handled high temperatures complex processes Carbon contamination and unintentional Hydrogen incorporation are sometimes a problem Schematics Basic transport and growth mechanisms Deposition process takes place on the substrates (wafers) Source https://en.wikipedia.org/wiki/Metalorganic_vapour_phase_epitaxy Step for MOCVD process Step 1. The atoms that we would like to be in our crystal are combined with a complex organic gas molecules and passed over a heated semiconductor substrate. Ga(CH3)3 + AsH3 (Trimethal gallium gas) (Arsene gas) Step 2. The heat break up the molecules and deposite the desired atoms on the surface layer by layer, e.g., Ga and As atoms on the substrate surface. 3CH4 + (Methane gas) GaAs (on the substrate Step 3. The atoms bond to the substrate surface and a new crystalline layer is grown, in this case GaAs, The reaction occurs in the chamber (reactor) Arsene gas is highly toxic & highly flammable! Trimethal gallium gas is highly toxic!! Methane gas is highly explosive! Kinematics reaction J1: molecular flux from the gas phase to the substrate surface, J2: consumption flux of GaAs corresponding to the surface reaction: J1 ≈ hG (CG – CS) J2 ≈ kSCS with hG = Gas phase mass transport coefficient, CG = gas-phase concentration, CS = Concentration on surface kS = Surface reaction rate Kinematics reaction In Steady-state conditions: J1=J2 That is v J1 J 2 cG 1 1 hG kS The deposition rate /growth rate of film is proportional to v is v Limiting cases: hG >> kS : Reaction Limited Growth kS >> hG : Transport Limited Growth cG 1 1 hG k S Reaction limited growth Small kS Growth controlled by processes on surface adsorption • decomposition • Surface reaction • chemical reaction • desorption of products kS kS is highly temperature dependent (increases with T) Common limit at lower temperatures Often preferred, slow but epitaxial growth Temperature and reactant choices are important Mass Transport Limited Growth Small hG Growth controlled by transfer to substrate hG is not very temperature dependent Common limit at higher temperatures Non-uniform film growth Gas dynamics and reactor design are important Material source should be sufficiently volatile high enough partial pressure to get good growth rates stable at room temperature produce desired element on substrate with easily removable byproducts Growth of III-V semiconductors: Group III: generally metalorganic molecules (trimethyl- or triethyl- species) Group V: generally toxic hydrides (AsH3; PH3 flammable as well); alternative: alkyls (TBAs, TBP). Desirable properties of precursors: • • • • • • • • Low toxicity Liquid at room temperature Suitable vapor pressure at room temperature Low carbon contamination in grown layer(avoid CH3radicals), however, for some applications C doping is desired No parasitic reactions with other sources Good long term stability (should not decompose in bubbler) Pyrolysistemperature should match growth temperature Inexpensive for industrial mass production Carrier gas should be “Inert” carrier gas constitutes about 90 % of the gas phase stringent purity requirements. H2 traditionally used, simple to purify by being passed through a palladium foil heated to 400 °C. Problem: H2 is highly explosive in contact with O2 high safety costs. Alternative precursor : N2: safer, recently with similar purity, more effective in cracking precursor molecules (heavier). High flux fast change of vapor phase composition. Regulation: mass flow controller Application …Application Laser diode: Transistors Solar Cells LED source how MOCVD works by AIXTRON Referance 1. 2. 3. 4. https://en.wikipedia.org/wiki/Metalorganic_vapour_phase_epita xy , 26/5/2013. Gerald B, Organometallic Vapor-Phase Epitaxy: Theory and Practice AIXTRON, how MOCVD works, Deposition Technology for Beginners Hugh O. Pierson, HANDBOOK OF CHEMICAL VAPOR DEPOSITION(CVD) Principles, Technology, and Applications Second Edition, NOYES PUBLICATIONS Park Ridge, New Jersey, U.S.A.