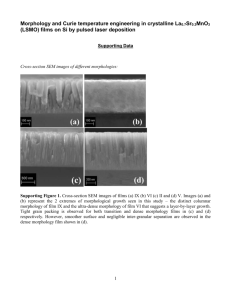
+ H 2 - Chemistry
advertisement

SYNTHESIS OF THIN FILMS FILMS - FORM? • Supported - substrate type - effect of interface – single crystal (oriented) or glassy • Free standing - synthetic strategy - air/liquid or liquid/liquid interface or substrate lift-off • Epitaxial - lattice matching and tolerance with underlying substrate • Superlattice - artificial multilayer – periodic or aperiodic • Patterned - chemical or physical lithography – bottomup synthesis or top-down physical methods FILMS - WHEN IS A FILM THICK OR THIN? • Monolayer - atomic, molecular thickness • Multilayer - compositional superlattice - scale - periodicity • Bulk properties - scale - thickness greater than l(e,h) • Quantum size effect - 2D spatial confinement – quantum confined along z, free electron behavior along x,y – called quantum wells – enable range of quantum devices THIN FILMS VITAL IN MODERN TECHNOLOGY • Protective coatings • Optical coatings - antireflection coatings • Electrochromic windows – photochromic spectacles • Dielectric film – low k circuit packaging – high k transistor gate insulation • Optical filters • Microelectronic devices and circuits • Optoelectronic devices and circuits • Photonic devices and circuits THIN FILMS VITAL IN MODERN TECHNOLOGY • Electrode surfaces – solar cells, fuel cells, lithum solid state batteries • Xerography, photography • Electrophoretic, electrochromic, electrodewettability displays • Catalytic and photocatalytic surfaces • Nanoporous membranes – gas separation • Information storage - magnetic, magnetoresistant, magneto-optical, optical, flash FILM PROPERTIES - ELECTRICAL, OPTICAL, MAGNETIC, MECHANICAL, ADSORPTION, PERMEABILTY, CHEMICAL • Thickness and Surface : Volume ratio • Surface vs bulk structure - surface reconstruction, dangling bonds – unsatisfied valencies, surface roughness • Hydrophobicity - hydrophilicy - wettability • Composition – surface vs bulk • Texture - single crystal, microcrystalline, orientation, glassy • Form - supported or unsupported (free-standing) nature of substrate - patterned or un-patterned METHODS OF SYNTHESIZING THIN FILMS • ELECTROCHEMICAL, PHYSICAL, CHEMICAL • Cathodic or anodic • Electroless deposition • Laser ablation • Cathode sputtering, vacuum evaporation, e-gun • Thermal oxidation, nitridation, sulfidation METHODS OF SYNTHESIZING THIN FILMS • ELECTROCHEMICAL, PHYSICAL, CHEMICAL • Liquid/melt phase epitaxy • Self-assembly - surface molecule anchoring, organization, close-packing, monolayers or multilayers • Discharge (plasma) techniques - RF, microwave • Chemical vapor deposition CVD, metal organic chemical vapor deposition MOCVD • Molecular beam epitaxy, supersonic cluster beams, aerosol deposition ANODIC OXIDATIVE DEPOSITION OF FILMS • Deposition of metal oxide films, such as alumina, titania by oxidation of metal electrode in aqueous salts or acids • Deposition of conducting polymer films by oxidative polymerization of monomer, such as thiophene, pyrrole, aniline, acetylene ANODIC OXIDATION OF Al IN OXALIC OR PHOSPHORIC ACID TO FORM ALUMINUM OXIDE • Pt|H3PO4, H2O|Al ECCell • Al Al3+ + 3e- Anode • PO43- +2e- PO33- + O2- Cathode • 2Al3+ + 3O2- g-Al2O3 (annealing) a-Al2O3 • Voltage control of oxide thickness • Al3+/O2- diffuse through growing layer of Al2O3 ANODIC OXIDATION OF PATTERNED Al DISC TO MAKE PERIODIC NANOPOROUS Al2O3 MEMBRANE SiC patterned master harder than Al to make nanoimprint replica How to remove residual Al and Al2O3 barrier layer??? 2Al + 3PO43- Al2O3 + 3PO332Al + 3C2O42- Al2O3 + 6CO + 3O2- ANODIC OXIDATION OF PATTERNED Al DISC TO MAKE PERIODIC NANOPOROUS Al2O3 MEMBRANE Aqueous HgCl2 dissolves Al to give Hg and Al(H2O)63+ and H3PO4 dissolves Al2O3 barrier layer to give Al(H2O)63+ - yields open channel membrane ANODIC OXIDATION OF LITHOGRAPHIC PATTERNED Al TO PERIODIC NANOPOROUS Al2O3 Not bad for chemistry!!! Hexagonal close packed nanochannel membrane ANODIC OXIDATION OF LITHOGRAPHIC PATTERNED Al TO PERIODIC NANOPOROUS Al2O3 40V Voltage control of channel diameter 50-500 nm accessible 60V 80V PROPOSED MECHANISM OF ALUMINA PORE FORMATION IN ANODICALLY OXIDIZED ALUMINUM SELF ORGANIZED SELF LIMITING GROWTH OF PORES Electric and strain fields guide and organize hcp channel growth Templated synthesis of metal barcoded nanorods Collection of multi-metal nanorods imaged in an optical microscope by the different reflectivity’s of different metal segments, Science 2001, 294, 137 • • Optical (A) and field emission scanning electron microscopy FE-SEM (B) images of an Au-Ag multistripe nanorods 550-nm Au stripes and Ag stripes of 240, 170, 110, and 60 nm -top to bottom 240 nm 550 nm 170 nm 110 nm 60 nm Orthogonal assembly on nanorods. Butyl isonitrile is bound non-selectively to Pt and Au surfaces. Aminoethanethiol displaces isonitriles selectively on gold but not on platinum. Rhodamine isocyanate is reacted with terminal amino groups to fluorescently label gold segments. -NH-CS-NHthiourea linkage of rhodamine fluorescent dye to Au segment DNA sandwich hybridization assay on metal barcode nanorods - Science 2001, 294, 137 SYNTHESIS OF CHEMICALLY POWERED NANOROD MOTORS ? Ozin et al Chem Comm, AdvMater 2006, Mallouk et al JACS 2005 ANODIC OXIDATION OF Si TO FORM POROUS Si: THROWING SOME LIGHT ON SILICON • Typical electrochemical cell to prepare PS by anodic oxidation of heavily doped p+type Si • PS comprised of interconnected nc-Si with H/O/F surface passivation • nc-Si right size for QSEs and red light emission observed during anodic oxidation – electroluminescence ELECTRONIC BAND STRUCTURE OF DIAMOND SILICON LATTICE • • • • • • band structure of Si computed using density functional theory with local density and pseudo-potential approximation diamond lattice, sp3 bonded Si sites VB maximum at k = 0, the G point in the Brillouin zone, CB minimum at distinct k value indirect band gap character, very weakly emissive behavior absorption-emission phonon assisted photon-electron-phonon three particle collision very low probability, thus band gap emission efficiency low, 10-5% SEMICONDUCTOR BAND STRUCTURE: CHALLENGE, EVOKING LIGHT EMISSION FROM Si • Effective Mass Approximation Rexciton ~ 0.529e/mo where e = dielectric constant, reduced mass of exciton mo = memh/(me + mh) • Note exciton size within the bulk material defines the size regime below which significant QSEs on band structure are expected to occur, clearly < 5 nm to make Si work REGULAR OR RANDOM NANNSCALE CHANNELS IN ANODICALLY OXIDIZED SILICON WAFERS • Anodized forms of p+type Si wafer • Showing formation of random (left) and regular (right) patterns of pores • Lithographic pretexturing directs periodic pore formation PORE FORMING PROCESS IN ANODICALLY OXIDIZED SILICON WAFERS • Basics of electrochemical cell - p+-Si wafer anode in contact with aqueous HF electrolyte – simplified electrochemistry: • • • Si Si(4+) + 4e Si(4+) + 6F(-) [SiF6]24H(+) + 4e 2H2 • Mechanism of natural self-limiting process for regular pore formation based on wider band gap of PS compared to bulk Si and respective redox potentials for anodic oxidation KEY ISSUES: ORIGIN OF PHOTO- AND ELECTROLUMINESCENE OF POROUS SILICON • Origin of luminescence key point- as bulk Si is indirect band gap semiconductor with very weak light emission • Models for light emission include quantumspatial confinement, siloxenes, and SiOH • Luminescent nc-Si structure requires SiO, SiH surface bonds - caps dangling bonds -removes killer traps in band gap • Size dependence of k, m selection rules, scaling laws determine light emission properties • Mechanical, photochemical, chemical stability are key factors for devices – safety too - care with humidity control and toxic silane evolved Si[H]surface + H2O SiH4 • Efficient e-h charge-injection required for practical LED MAKING NANOCRYSTALLINE SILICON LUMINESCENT: CAPPING *(SiH) CB CB VB VB capping Si cluster dangling bond with H, F, O forms bonding-antibonding SiH -orbitals, moves killer trap states out of the gap facilitates radiative over non-radiative relaxation (SiH) Sin HxSin LIGHT WORK BY THE SILICON SAMURAI WHERE IT ALL BEGAN AND WHERE IT IS ALL GOING??? FROM CANHAM’S 1990 DISCOVERY OF PL AND EL ANODICALLY OXIDIZED p+-DOPED Si WAFERS, TO NEW LIGHT EMITTING SILICON NANOSTRUCTURES, AND DREAM OF SILICON OPTOELECTRONICS AND PHOTONIC COMPUTING – ACTUALLY BIOSENSORS EVENTUALLY CHEMICAL VAPOUR DEPOSITION • Pyrolysis, photolysis, chemical reaction, discharges RF, microwave facilitated deposition processes • Epitaxial films, correct matching to substrate lattice • CH4 + H2 (RadioF, MicroW) C, diamond films (perfect non-stick frying pan – inert, hard, transparent, non-stick, high thermal conductivity) • Et4Si (thermal, air) SiO2 • SiCl4 or SiH4 (thermal T, H2) a-H:Si or nc-H:Si • SiH4 + PH3 (RF) n-Si (ppm P) CHEMICAL VAPOUR DEPOSITION • Si2H6 + B2H6 (RF) p-Si (ppm B) • Single source precursor SiH3SiH2SiH2PH2 (RF) n-Si • Me3Ga (laser photolysis, heating) Ga • Me3Ga + AsH3 + H2 (T,P) GaAs + CH4 • Si (laser evaporation, molecular beam, high to low P supersonic jet, ionization) Sin+ (size selected MS - cluster deposition) Si H H H H H H H H HH H H H H H H H H H H H H H H H H H H H H H H H H H Amorphous hydrogenated silicon a-H:Si, easy to form thin film by CVD Hydrogen capping of dangling surface sp3 bonds Reduces surface electron killer traps Enhances electrical conductivity compared to a-Si but less than bulk c-Si Poly-domain texture Useful for pn and pin junction large area solar cell devices REMOVING DANGLING BONDS BY Si-H CAPPING *(SiH) CB CB VB VB capping Si cluster dangling bond with H, F, O forms bonding-antibonding SiH orbitals, moves killer trap states out of the gap facilitates charge transport and radiative relaxation (SiH) Sin HxSin METAL ORGANIC CHEMICAL VAPOR DEPOSITION MOCVD • Invented by Mansevit in 1968 • Recognized high volatility and chemical reactivity of metal organic compounds as sources for semiconductor thin film preparations • Enabling chemistry: electronic, optical quantum devices • Quantum wells and superlattices • Occurs for 5-500 Angstrom layers • Known as artificial superlattices Schematic energy band diagram of a quantum well structure showing confined electron and hole states produced by large Eg GaAlAs layers sandwiching small Eg GaAs depicting quantum size effects and some possible optical transitions CB edge GaAlAs CB/VB edges GaAs L En = n2p2h2/2m*L2 VB edge GaAlAs METAL ORGANIC CHEMICAL VAPOR DEPOSITION, MOCVD • Quantum confined electrons and holes when thickness of quantum well L is comparable to the wavelength of an electron or hole at the Fermi level of the material, band diagram shows confined particle states and quantization effects for electrical and optical properties • Discrete electronic energy states rather than continuous bands, given by solution to the simple particle in a box equation, assuming infinite barriers for the wells, m* is the effective mass of electrons and holes • En = n2p2h2/2m*L2 • Tunable thickness, tailored composition materials, do it yourself quantum mechanics materials for the semiconductor industry METAL ORGANIC CHEMICAL VAPOR DEPOSITION, MOCVD • Quantum well structure synthesized by depositing a controlled thickness superlattice of a narrow band gap GaAs layer sandwiched by two wide band gap GaxAl1-xAs layers using MOCVD • Ga(Al)Me3 + AsH3 (H2, T) Ga(Al)As + CH4 • Artificial superlattices, designer periodicity of layers, quantum confined lattices, thin layers, epitaxially grown, x determines electronic band gap • Example: GaxAl1-xAs|GaAs|GaxAl1-xAs MOCVD • Example: GaxAl1-xAs|GaAs|GaxAl1-xAs • n- and p-doping achievable by having excess As or Ga respectively in a GaAs layer • Composition and carrier concentration controls refractive index (low RI cladding, TIR optical confinement) and electrical conductivity (p-n and p-n-p junction devices), in a semiconducting superlattice • Enables electron (quantum) and photon (RI) confinement for electronic, optoelectronic and optical devices • Multiple quantum well laser, quantum cascade laser, distributed feedback laser, resonant tunneling transistor, high electron mobility ballistic transistor (HEMT), laser diode Resonant tunneling transistor BAND GAP ENGINEERING OF SEMICONDUCTORS • MOCVD, LPE, CVD, CVT, MBE all deposition techniques that provide angstrom precise control of film thickness • Together with composition control one has a beautiful synthetic method for fine tuning the electronic band gap and hence most of the important properties of a semiconductor quantized film BAND GAP ENGINEERING OF SEMICONDUCTORS • Key is to achieve epitaxial lattice matching of film with underlying substrate • Avoids interfacial lattice strain, elastic deformation, misfit dislocations, defects - all of these problems serve to increase carrier scattering, decrease charge-transport, increased quenching of e-h recombination luminescence (killer traps), thereby reducing the efficacy of the material for advanced device applications MOCVD SINGLE SOURCE PRECURSORS • Me3Ga, Me3Al, Et3In (synthesis GaCl3 + MeLi/R2Mg/RMgI) • NH3, PH3, AsH3 (synthesis Mg3As2/HCl) • H2S, H2Se • Me2Te, Me2Hg, Me2Zn, Me4Pb, Et2Cd • E.g. synthesize an IR detector based on p-n photodiode • Me2Cd + Me2Hg + Me2Te (H2, 500oC) HgxCd1-xTe • p-HgxCd1-xTe/n-HgxCd1-xTe • • p- and n-doping requires precise control of Hg/Cd and Te stoichiometry x determines the electronic bandgap – tuned to IR wavelength range for detector • Toxic materials – safe handling and disposal of toxic waste!!! Schematic of cold wall MOCVD system Single crystal substrate on inductively heated or resistively heated susceptor – mass flow control of precursors MOCVD deposited film H2/AsH3/PH3 Water cooling H2/InMe3/GaMe3 Thermocouple H2/PEt3 Waste gases H2/n-dope H2S/p-dope ZnMe2 MOCVD surface chemistry of precursors, nucleation and growth of product film on substrate CH4 Me Me Me Ga Me Me Me Me Ga Me H Me Al H H H As As Al As Al As Al As Al As Al As Al As Al As Al As Al As Precursor adsorption on single crystal oriented substrate - lattice matching epitaxy criteria - surface physisorption - chemisorption surface diffusion - dissociative chemisorption - reaction - desorption Different models for film nucleation and growth - depends whether surface diffusion involved - fixed vs mobile crystal nuclei MOCVD SINGLE SOURCE PRECURSORS • Specially designed MOCVD reactors, hot and cold wall designs, controlled flow of precursors using digital mass flow meters directing precursors to heated single crystal substrate, induction or resistive heater, silicon carbide coated graphite susceptor for mounting substrate • This chemistry creates problems for semiconductor manufacturers wrt safe handling and disposal of toxic waste • Most reactions occur in range 400-1300oC, complications of diffusion at interfaces, disruption of atomically flat epitaxial surfaces/interfaces may occur during deposition • Photolytic processes (photoepitaxy) help to bring the deposition temperatures to more reasonable temperatures PHOTOEPITAXY Making atomically perfect thin films under milder and more controlled conditions Et2Te + Hg + H2 (h, 200oC) HgTe + 2C2H6 Bottom graphite, middle substrate, top HgTe film H2 gas window Hg pool H2/Et2Te Exhaust gases UV illumination PHOTOEPITAXY Making atomically perfect thin films under milder and more controlled conditions • Mullin and Tunnicliffe 1984 • Et2Te + Hg (pool) + H2 (h, 200oC) HgTe + 2C2H6 • Et2Te/Me2Cd + Hg (pool) + H2 (h, 200oC) HgxCd1-xTe + 2C2H6 • MOCVD preparation requires 500oC using Me2Te + Me2Hg/Me2Cd • Advantages of photo-epitaxy • Lower temperature operation, multi-layer formation, less damage of layers - ternaries HgxCd1-xTe, n- and p-doping, Te and Hg/Cd rich, p-n diodes, IR photodetectors, multi-layers, quantum size effect devices HgxCd1-xTe|HgTe|HgxCd1-xTe PHOTOEPITAXY Making atomically perfect thin films under milder and more controlled conditions • Lower interlayer diffusion, easy to fabricate multilayers • Abrupt boundaries, less defects, strain and irregularities at interfaces • Note that H2 gas window in apparatus prevents deposition of HgTe on observation port • In this way CdTe can be deposited onto GaAs at 200250oC even with a 14% lattice mismatch • Key consideration - GaAs is susceptible to damage under MOCVD conditions 650-750oC REQUIREMENTS OF SUCCESSFUL MOCVD PRECURSOR • RT stable • No polymerization, decomposition • Easy handling • Simple storage • Not too reactive • Vaporization without decomposition REQUIREMENTS OF MOCVD PRECURSORS • Vaporization without decomposition • Modest < 100oC temperatures • Low rate of homogeneous pyrolysis, gas phase, wrt heterogeneous, surface, decomposition • HOMO : HETERO rates ~ 1 : 1000 • Heterogeneous reaction preferred on substrate • Greater than on other hot surfaces in reactor REQUIREMENTS OF MOCVD PRECURSORS • Not on supports or reaction chamber/vessel • Ready chemisorption of precursor on substrate • Detailed surface and gas phase studies of structure of adsorbed species, reactive intermediates, kinetics, vital for quantifying film nucleation and growth processes • Electrical, magnetic, optical films made in this way • Semiconductors, metals, silicides, nitrides, oxides, mixed oxides (e.g., high Tc superconductors), sulfides, selenides CRITICAL PARAMETERS IN MATERIALS PREPARATION FOR SYNTHESIS OF THIN FILMS • Composition control - precise command over stoichiometry and adventitious carbonaceous deposits • Variety of materials to be deposited • Good film uniformity • Large areas to be covered, > 100 cm2 • Precise reproducibility CRITICAL PARAMETERS IN MATERIALS PREPARATION FOR SYNTHESIS OF THIN FILMS • Growth rate, thickness control • 2-2000 nm layer thickness • Precise control of film thickness • Accurate control of deposition, film growth rate CRITICAL PARAMETERS IN MATERIALS PREPARATION FOR SYNTHESIS OF THIN FILMS • Crystal quality, epitaxy • High degree of film perfection • Defects degrade device performance • Reduces useable wafer yields CRITICAL PARAMETERS IN MATERIALS PREPARATION FOR SYNTHESIS OF THIN FILMS • Purity of precursors • Usually less than 10-9 impurity levels • Stringent demands on starting material purity • Chemistry challenge, purifying, analyzing precursors at ppb level • Demands exceptionally clean growth system otherwise defeats the object of controlled doping of films for device applications CRITICAL PARAMETERS IN MATERIALS PREPARATION FOR SYNTHESIS OF THIN FILMS • Interface widths • Abrupt changes of composition, dopant concentration required, vital for quantum confined structures • 30-40 sequential layers often needed • Alternating composition and graded composition films • 0.5-50 nm thickness required with atomic level precision • All of the above has been more-or-less perfected in the electronics and optics industries – amazing achievement!!! III-V BAND GAP ENGINEERING 1500nm • • • • • • • • • • • • Designer semiconductors Single crystal substrate Single crystal layers Zinc blende lattice Lattice constant Composition Doping Thickness Multilayers Epitaxial lattice matching Control of Eg band gap and RI refractive index Operating wavelengths for optical telecommunication systems labeled in purple TECHNIQUES USED TO GROW SEMICONDUCTOR FILMS AND MULTILAYERED FILMS • MOCVD • Liquid phase epitaxy • Chemical vapor transport • Molecular beam epitaxy • Laser ablation • Used for band gap engineering of semiconductor materials that function at 1.5 microns in near IR integrating with glass fiber optics and waveguides 6InP/3GaAs/6InP EPITAXIALLY MATCHED SUPERLATTICE TAILORED BAND GAPS - DESIGNER MOCVD GRADED COMPOSITION POTENTIAL WELLS AlxGa1-xAs graded composition-gap superlattice e Tunable h h CB AlAs wide gap CB GaAs narrow gap VB GaAs narrow gap VB AlAs wide gap Designer quantum well architecture - band gap engineering - graded composition enables gradient potential – speeds mobility of electrons injected into channel - used to enhance performance in high electron mobility transistors HEMTs or build a quantum cascade laser Federico Capasso co-inventor of the quantum cascade laser imagined small things when he used size and dimensionality of materials to tailor their properties for electronic and optical devices QUANTUM CASCADE LASER - A NICE EXAMPLE OF BAND GAP ENGINEERING BY MOCVD h h h White bands in the TEM are QWs made of narrow band gap GaInAs, which are sandwiched between barrier layers of wide band gap AlInAs ranging in thickness from atomic to 12 atomic layers All wells are part of a QCL Voltage applied to device, electrons move down potential barrier from wide to narrow Eg QWs (tunnel from QW to QW) and emit a photon between two thickest QWs. Electrons move on (tunnel) to the next stage to the right where the process repeats – hence cascade laser.