Absorption on the surface - physisorption and Chemisorption
advertisement
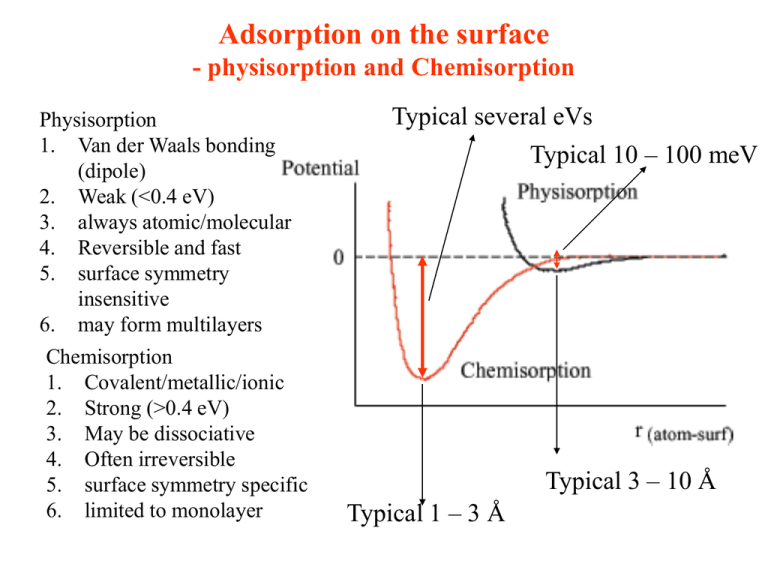
Adsorption on the surface - physisorption and Chemisorption Physisorption 1. Van der Waals bonding (dipole) 2. Weak (<0.4 eV) 3. always atomic/molecular 4. Reversible and fast 5. surface symmetry insensitive 6. may form multilayers Chemisorption 1. Covalent/metallic/ionic 2. Strong (>0.4 eV) 3. May be dissociative 4. Often irreversible 5. surface symmetry specific 6. limited to monolayer Typical several eVs Typical 10 – 100 meV Typical 3 – 10 Å Typical 1 – 3 Å Chemisorption It is chemical reaction, like the chemical bonding in the molecules. Schematic drawing show covalent chemisorption bonding between a molecule and a transistion metal (d bands dominate at Fermi surface). The rearrangement of the electronic shell during chemisorption may lead to physisorption dissociative dissociative adsorption adsorption Work Function change induced by adsorption Different material have different f. Moreover, chemisorption change the charge distribution and cause f change. For semiconductor even band bending is changed. The dipole of the adsorbate can change f. Under different condition (T for example), different adsorption bonding for the same adsorbate can be formed on the same surface with different f. Phase diagram for 2 D adsorbates When without long range order then is 2D liquid droplets. Random, dilute phase of 2D gas 2D crystallites with internal order The relative strength of the lateral and vertical interactions determines if an ordered adsorbate layer is with registry with the substrate. The lateral ones can be: van der Waals attraction, dipole forces (between parallel ones, repulsive), the repulsive force due to orbital overlap between neighbors, substrate-mediated interactions (modification of electronic structure, elastic properties to compensate the change of the lattice.) Phase diagram for 2 D adsorbates Describe the inter-particle potential For 3D system, van der Vaals equation ( p an2 )(1 nb) nKT a 2 1 For 2D system: ( Splay pressure d )( f p ) kT Area density Minimum area of particle Adsorption Kinetics Adsorption rate: Coverage then: dN p uS S dt 2m kT dN udt S dt Or dt S 2mkT / p In case for activated adsorption, S as function: S ( ) f ( ) exp(Eact / kT ) Condensation coefficient Occupation factor d dt Adsorption isotherm Together with adsorption process, there is desorption process which can be described by description rate: v ( ) f ( ) exp(Edes / kT ) Occupation factor Desorption efficient Adsorption isotherm describe the equilibrium adsorbate coverage at certain temperature between adsorption and desorption: u = v More f ( ) 1 f ( ) complcated p 2m kTe EB / kT f ( ) A f ( ) when adsorbate For non-dissociative adorpstion: f()=1- atoms allow And f()=. Therefore, adsorption Ap ( p) 1 AP Curves tell the strength of the adsorption Surface Diffusion The adsorbate atoms on the surface although lost some kinetic energies to the substrate after adsorption, it can vibrate in potential well with frequency n0 and also hop (surface diffusion) from one site to the neighbor site with frequency n: 1 n n 0 exp( E (diff ) / kT ) z z is the number of possible neighboring wells. Ordered monolayers can only form if surface diffusion is high. Typically, ~ 1013 s-1 and E(diff) 100-300 kJ·mol-1 Film epitaxy The expression “epitaxy” has been introduced on a geometrical basis. It characterizes the oriented growth of a material A on top of a crystalline substrate B. If A and B are the same material, the growth is called homoepitaxy. Otherwise, it is called heteroepitaxy. For equilibrium condition Bauer’s criteria: Dg=ga+gab-gb Dg < 0 Layer-by-layer growth (Frank-van der Merwe (FM)) Dg > 0 Island growth (Vollmer-Weber mode) Dg is function of thickness, initially Dg < 0 then Dg > 0 Transition layer/island growth(Stranski-Krastanov mode) Growth kinetics the microscopic kinetic processes taking place at the surface: • Adsorption from the vapor phase • Surface diffusion followed by binding or re-desorption • Nucleation of 2-dimensional or 3-dimensional cluster • Capture by an existing cluster or surface defect sites (steps) • Interdiffusion with the substrate in most cases the growth are done at relatively low temperature and high deposition rate. Even thermodynamics favors 3-dimensional islands formation, monolayer-like growth can be approached by reducing atomic mobility and so that the equilibrium shape cannot form. The growth modes also depends on defects. Surfactant-to achieve FM growth The interlayer mass transport is a prerequisit for layer-by-layer growth, however, the potential barrier can prevent such mass transport. Surfactant is a contaminant which, when deposited once, promotes a smoother growth. Possible mechanisms: •Surfactant decorate edges and reduce the step-edge barrier •It induce a potential energy gradient to attract deposited atoms toward the steps. •It reacts as nucleation centers by attracting adatoms to increase the island density. •Surfactant atoms are practically immobile on the surface and act repulsively to deposited diffusing adatoms. Some examples for surfactant The comparison between growth of Cu on Ru(0001) with/without Oxygen as surfactant, Cu on Cu(111) with/without Pb. Phys. Rev. Lett. 81, 850-853 (1998) Growth techniques As the kinetics process is important to growth, the techniques have their distinct important factors: Deposition temperature, rate, energy of the particles deposited, pressure, vacuum requirement, substrate morphology, etc. Molecular Beam Epitaxy (MBE) Typical MBE chamber In MBE, the constituent elements in the form of “molecular beams” are deposited onto a crystalline substrate to form thin epitaxial layers. The “molecular beams” are typically from thermally evaporated elemental sources. Knudsen Effusion Cell basis of nearly all beam generation. The cells contain the condensed phase and its vapor in equilibrium. For a cell of orifice area, A, a distance l from the substrate, and at temperature T(K), the flux of molecules or atoms striking a unit are of substrate : J = (1.118x1022)pA/[l2(MT)1/2] molecules cm-2s-1 where p is the source pressure in the cell in Torr, and M is the Molecular weight of the source material.Therefore T essentially determines the rate and need to be controlled. The distribution across a flat substrate for such a cell can be expressed as: J = Jo cos4 Knudsen Effusion Cell Normally the materials in the cell need to be heated to increase the vapor pressure till 10-3 Torr to get a reasonable growth in the high vacuum (typical rate 1 m/h or 200 Å/min). Therefore, from the temperature dependence of the equilibrium vapor pressure of certain material, one can already determine a suitable temperature for MBE growth of this particular material. If the temperature is too high, the growth by MBE will be very difficult or even practically impossible and other methods need to be considered. Normally the cell is heated by resistive way, but some new types can use e-beam with high voltage. cell Electron Beam (EB) source For the low-vapor-pressure materials, electron beam (EB) with high voltage is used to heat the source materials to reach very high temperature for evaporation. No crucible is necessary and can fast reach extremely high temperature. Magnetron Sputtering (MS) Sputter sources for film deposition can be categorized in two ways: glow discharge and ion beam. Magnetron Sputtering belongs to the first category. In all cases, atoms are removed from the target by momentum transfer from the incident ions with a large accelerating energy to the surface atoms of target material, then the sputtered atoms for the film growth. (c) The plasma is important for glow discharge sputtering process and is typically formed by partially ionizing a gas at a pressure well bellow atomspheric (normally from 0.1 to 1000 mtorr). For the most part, these plasmas are very weakly ionized, with an ionization ration of 10-5 to 10-1. Plasmas are generally neutral, in that in the body of the plasma there are roughly equal numbers of electrons and ions. They are conductive with the dominant charge carriers: the electrons. There are generally three types for plasma generation: excited between two powered electrode; application of electric fields, typically through an insulator; injection of large currents of electrons to ionize the gas particle. Dc/rf sputtering Dc diode sputtering with typical voltages 3-5 kV and a current from 50-250 mA at a pressure of 50-250 mtorr. For deposition purpose, the target material will be use as cathode and samples are placed on the anode or nearby. It is slow, and it needs high gas density, high discharge voltage, and conductive target as the cathode (no insulator for dc sputtering). Rf sputtering is more easily to be used with low gas density and low voltage, and works with insulator too. The reason is the quickly change polarity of the E field with MHz, the electron will quickly react and to compensate any charging on the sample surface, while the ion with big mass is still sputtering. The bias in RF sputtering Due to the rather bigger mass than electron, the powered electrode will in the first several cycles collect more electrons than ions, therefore, there will be a negative dc bias generated, which prevent electrons be further collected and only ions will be accelerated by the dc bias toward the electrode (the material needed to be sputtered) and less influenced by rf field. This bias is just half of the voltage of rf oscillation. Magnetron Sputtering (MS) Charged particles will have drift motion in the presence of E and B with drift velocity: Vdrift = E/B Electrons circles in a too large circles compared with chamber size, but ions will circle and drift inside the chamber. Magnetron is to keep ions circles over the target to be easily generate plasma. Pulse Laser Deposition (PLD) Pulsed laser radiation is used vaporize materials and collect the vapor onto a substrate. The laser-solid interaction leads to evaporation, ablation, plasma formation, and exfoliation. The plume generated by the laser beam consists of mixture of energetic species including atoms, molecules, electrons, ions, clusters and even micron-sized particulates. Pulse Laser Deposition (PLD) Schematic diagram for three fluence levels on the target surface. (a) At low fluence ripple formation. (b) At higher fluence, surface uniformly melted and larger scale capillary waves develop. (b) At PLD fluence, melt depth is greater and vaporization occurs, and capillary waves still form This change of morphology will strongly influenced the stability of the growth rate. A series of plots of deposition rate versus exposure for different YBCO target density. They all show a clear decay. Summary of the three deposition techniques Techniques (Torr) MBE MS PLD Energy(eV) 0.1 1-1000 1-1000 rate (Å/s) 0.01-1 1-100 1-100 Vacuum Operation pressure UHV HV HV 10-9 10-1 and 10-3 10-9 to 10-1 MBE: good control of film (purity and thickness), too slow, difficulties in multi-components. MS: large area evaporation, fast growth, workable with reactive gas, impurities, the inefficient usage of the targetmaterial, difficulties in magnetic targets PLD: keep stoichiometry, almost every material target, super-high instant growth rate, reactive gas, easy to change targets, particulates and the difficulty to grow in large area Thickness calibration a. The intensity of MEED (medium energy electron diffraction) and RHEED, or even other scattering methods like Atom scattering is sensitive to surface roughness, which can be used to monitor the thickness during growth for layer-by-layer mode. b. Auger/XPS/absorption, etc, have certain free path length, which can be used to study the thickness. c. Quartz crystal deposition monitor uses the piezoelectric sensitivity of a quartz monitor crystal to added mass, which corresponds to film thickness when provided known density. Auger/XPS ratio 1. Think about the mean free path length of the electrons. 2. Think about the coverage (morphology) Chemical Vapor Deposition (CVD) Techniques thin films are deposited on a substrate from vapor phase precursors through chemical reactions at the surface, CVD involves many coupled processes, including fluid flow, heat and mass transfer, chemical kinetics, and nucleation and growth of the film on the substrate CVD versus physical depostion •Compared to other deposition techniques the CVD method is perhaps the most complex. •Unlike growth by physical deposition such as evaporation or MBE, this method requires numerous test runs to reach suitable growth parameters, especially for single-crystal growth. •The complexity of this method results from the facts that: (i) it generally includes multicomponent species in the chemical reactions, (ii) the chemical reactions generally produce intermediate products, (iii) the growth has numerous independent variables, and (iv) the growth includes more consecutive steps than in physical methods. CVD process – CVD—thermally activated (or pyrolytic) chemical vapour deposition; – MOCVD—metalorganic CVD; – PCVD—photo CVD; – PECVD—plasma-enhanced CVD; – ALE—atomic layer epitaxy. Technique Energy promoting the reactions What is specific? Pressure (Torr) (Thermal) CVD Thermal Inorganic sources 10- 5 –760 MOCVD Thermal Organometallic sources 10–760 PECVD Plasma Lower temperatures 10–760 PCVD Light Lower temperatures, selected area 0.01–10 ALE Thermal/Light monolayer control 0.01–760