Covalent Bond
advertisement

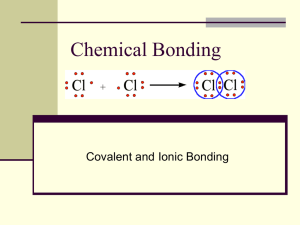
Periodic Table Study Guide How to Draw Lewis Structures Lewis Structures 1) Find your element on the periodic table. 2) Determine the number of valence electrons. 3) This is how many electrons you will draw. Lewis Structures • Find out which group (column) your element is in. • This will tell you the number of valence electrons your element has. • You will only draw the valence electrons. www.chem4kids.com Groups - Review Group 1 = 1 electron Group 2 = 2 electrons Group 8 = 8 electrons Except for He, it has 2 electrons •Each column is called a “group” •Each element in a group has the same number of electrons in their outer orbital, also known as “shells”. www.chem4kids.com •The electrons in the outer shell are called “valence electrons” Location of valence electrons in a Lewis Dot Structure • How many total electrons can exist in the valence shell of H and He? • How about Li and heavier? • Follow my lead! Lewis Dot Structures: Group 2A Example: Beryllium 1) Write the element symbol. 2) Beryllium is in the 2nd group, so it has 2 valence electrons. 3) Starting at the right, draw 2 electrons 4) (or dots). What other elements have the same Lewis Dot Structure? Why? Lewis Dot Structures: Group 3A Example: Boron 1) Check your work. 2) Using your periodic table, check that Boron is in the 3rd group. 3) You should have 3 total electrons, or dots, drawn in for Boron. Lewis Dot Structures: Group 5A Example: Nitrogen On your worksheet, try these elements on your own: a) b) c) d) e) f) H P Ca Ar Cl Al Lewis Structures On your worksheet, try these elements on your own: a) b) c) d) e) f) H P Ca Ar Cl Al Lewis Structures On your worksheet, try these elements on your own: a) b) c) d) e) f) H P Ca Ar Cl Al Lewis Dot Structures On your worksheet, try these elements on your own: a) b) c) d) e) f) H P Ca Ar Cl Al Lewis Structures On your worksheet, try these elements on your own: a) b) c) d) e) f) H P Ca Ar Cl Al Lewis Structures On your worksheet, try these elements on your own: a) b) c) d) e) f) H P Ca Ar Cl Al Lewis Structures On your worksheet, try these elements on your own: a) b) c) d) e) f) H P Ca Ar Cl Al Lewis Dot Structures – Carbon Family • The elements in the Carbon family are unique • The stability of a an atom in the carbon family is special. • Hund’s Rule: An atom with a half filled shell is more stable than a partially filled shell. • This goes back to the electron configuration of Carbon. • 6 total electrons What you were told to do: ___ ___ ___ ___ ___ 1s 2s 2p What actually happens: ___ ___ ___ ___ ___ 1s 2s 2p Lewis Dot Structure for Carbon Family Bohr Model of First 20 elements Complete the Lewis Structure Worksheet You should know how to draw Lewis Structures for the first 20 elements. Ionic Charge Atoms that gain or lose electrons become ions. • Atoms with low electronegativity will lose electrons – Example: Metals • Atoms with high electronegativity will gain electrons – Example: Nonmetals An atom will gain or lose electrons to become more stable by having a full valence shell – “The octet rule” • Ionic charge is the net electric charge that an atom will have after it gains/loses electrons to have a full valence shell. Label the following as atoms or ions Net Charges What is the net charge of this atom? What is the net charge of these ions? Ionic Charges of the Representative Elements Ion Representation Cations Anions • A cation is formed when an atom gives an electron. • The cation has more protons than electrons • So it has a net positive charge. Example: Sodium atom to Sodium Ion • An anion is formed when an atom gains an electron • The anion has more electrons than protons • So it has a net negative charge. Example: Fluorine atom to Fluorine ion Na [Na]1+ F [ F ]1− Fill in the electrons and show the gained electron with another symbol Practice: Ionic Charge Directions • Draw the Lewis dot structure • Show electrons that are gained or lost • Determine ionic charge • Represent as an ion Examples: 1. Magnesium 2. Phosphorus Review Questions: 1. Ionic bonds occur between __________ and __________. 2. When atoms lose electrons they gain a ______ charge. 3. When atoms gain electrons they gain a ______ charge. 4. Atoms that gain or lose electrons are called ________. 5. An ionic bond occurs when atoms ______ or ________ electrons. Chemical Bonding Ionic Bonding (form salts) Covalent Bonding (form molecules) Ionic Bonds: Covalent Bonds: http://www.youtube.com/wat • http://www.youtube.com/w ch?v=T40sM8-SXso atch?v=0mUncUj55FI Naming Ionic Bonds http://www.youtube.com/wat ch?v=7Lfc6jjp1WQ Bonding: http://www.youtube.com/watch?v= QqjcCvzWwww Naming Covalent Bonds: • http://www.youtube.com/w atch?v=VokWJy_jpAc Example of 1:1 ratio: Ionic bonds Salt: Li + F Practice: Ionic Bond formulas Reaction K + Cl Na + I Name Example of 1:2 ratio: Ionic bonds Salt Ca + C Practice: Reaction Mg + Cl Na + S Name Example of 1:3 ratio: Ionic bonds Salt Ga + I Example of 3:2 Ratio: Ionic Bonds Name Covalent Bonding Covalent Bond • A bond formed when atoms share one or more pairs of electrons • Compounds that are made of molecules have covalent bonds • Covalent bonds usually form between two non-metals. Covalent Bonding Covalent Bonds: • http://www.youtube.com/w atch?v=0mUncUj55FI Naming Covalent Bonds: • http://www.youtube.com/w atch?v=VokWJy_jpAc Now, Read The Bare Essentials of Polarity comic strip and answer the questions on the question guide. Molecular Polarity Non polar Polar (dipole molecules) • When electrons are equally • When electrons are not attracted to the positive equally attracted to the nucleus of each atom the positive nucleus of each bond is NON-POLAR atom the bond is POLAR. • The molecule will not have • The molecules will have charged ends (called poles) oppositely charged ends (called poles) • Example: Diatomic molecules • Example: Water Electronegativity nonpolar covalent, polar covalent, and ionic bonds • http://www.youtube.com/watch?v=Kj3o0Xvh VqQ Polar vs. Nonpolar Molecules Polar Molecules: Water Polar molecules: water The Role of Electronegativity Remember: The electronegativity of an atom is its attraction for another atoms valence electrons. Brightstorm video: http://www.youtube.com/watch?v=un93VJxUfPA Covalent Bonding Diatomic Molecule: Single Bond (nonpolar) Covalent Boding Diatomic Molecules: Double Bond (Nonpolar) Covalent Bonding Diatomic molecule: Triple Bond (nonpolar) Circle the Full valence shell of electrons How many total bonds? Are these bonds polar or nonpolar? Indicate the single, double and triple bonds. How many total electrons are being shared? Lewis dot Structure and Formula for a Covalent Bond Circle the valence electrons for each Representing Elements and Covalent bonds with Lewis structures Covalent bonding practice sheets