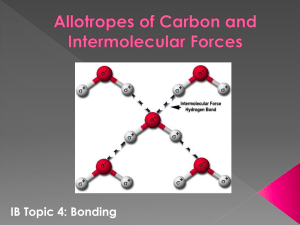
Intermolecular Forces
advertisement

Intermolecular Forces HONORS CHEMISTRY JANUARY 2011 Corresponds to “Short Notes on Intermolecular Forces Worksheet” Classifying the molecule First classify the molecule: Ionic (M+NM) or Covalent (NM +NM) Polar or NonPolar for Covalent Molecules Then determine the force of attraction: Electrostatic Forces – ionic compounds Hydrogen Bonding – H bonded to O, N, or F Dipole-Dipole Forces – Polar covalent molecules London Dispersion Forces (LDF) – NonPolar covalent molecules Question 1: What is the force of attraction between molecules of the following substances? a) PCl3 – polar covalent compound; therefore dipole-dipole forces b) NiCl2 – ionic compound - Ni2+ and Cl-; therefore electrostatic forces c) I2 – nonpolar covalent compound; LDF d) HF – covalent compound; H is bound to F therefore Hydrogen e) f) g) h) Bonding HCl – covalent compound; H is bonded to Cl therefore dipole-dipole forces CH2O – covalent compound with both H’s and O bonded to Carbon. NOT Hydrogen bonding because C is bonded to O. However, it does have dipole-dipole forces H3PO4 – covalent molecule where the H is bonded to the O; therefore Hydrogen Bonding BF3 – covalent compound, trigonal planar with the molecules same on the all side making the molecule nonpolar; therefore LDF Ranking the Strength of Intermolecular Forces In general: strongest weakest Electrostaic H bonding dipole-dipole forces LDF 2. Rank the strength of the following compounds from strongest to weakest: PCl3, NiCl2, I2, HF NiCl2 > HF > PCl3 > I2 NiCl2 is an ionic compound with electrostatic forces; therefore it is the strongest HF – hydrogen bonds (2nd strongest) PCl3 – dipole-dipole forces I2 – LDF (weakest) Compounds can be ranked within the category of Electrostatic Forces: Compounds made of ions with higher charges will be STRONGER than ions with lower charges. Smaller ions are STRONGER than larger ions. Ions become larger as you go down the group 3. Rank the following ionic compounds according to the strength of their electrostatic forces of attraction: NiCl2, Fe2O3, MgO, KI, BeF2, CrCl3 Fe2O3 > MgO > CrCl3 > BeF2 >NiCl2 > KI First, look at the charges that make up the compounds. Fe3+, O2Mg2+, O2Cr3+, ClBe2+, FNi2+, ClK+, I- Then notice that MgO is greater than CrCl3 even though +3 > +2. Also, notice that NiCl2 and BeF2 have the same charges; therefore you look at the size. Be and F are in the second period which makes them smaller than Mg and O (3rd and 4th period). The smaller ions have stronger forces. Compounds can be ranked within the category of London Dispersion Forces: These forces are due to a temporary dipole; therefore the molecules with bigger electron clouds will more easily and more greatly perturbed. The number of electrons is related to the number of protons, which is related to molar mass. Nonpolar compounds with higher molar masses will have STRONGER London Dispersion Forces. 4. Rank the following from strongest to weakest intermolecular forces: Cl2, CH4, BF3, SCl2, CO2 SCl2 > Cl2 > BF3 > CO2 > CH4 All of the molecules are nonpolar except SCl2 which has dipole-dipole forces of attraction. Rank the others in order of Molar Mass. Cl2 70.91 g/mol BF3 67.81 g/mol CO2 44.01 g/mol CH4 16.05 g/mol Ranking Physical Properties Predictions about various physical properties can be made based on the strength of the intermolecular forces. Solubility Melting Point Boiling Point Vapor Pressure of Pure Substances Viscosity and Surface Tension Solubility Forces between the solvent and the solute must overcome the solute-solute and solvent-solvent forces. Most effective if forces are similar “Like dissolves like.” Example: Polar molecules can dissolve into polar solvent. Example: Nonpolar molecules can dissolve into nonpolar solvent. Determine solubility of ionic compounds from the solubility rules in book/reference sheet. Water is most common solvent. It is polar. 5. Which of the following compounds are likely to dissolve in water? a) SCl2 – polar molecule; therefore YES dissolves in water b) O2 – nonpolar molecule; NOT soluble in water c) NaCl – ionic compound. It has low charges (Na+ and Cl-) and according to the solubility rules “group I cations are always soluble”; therefore YES soluble in water. d) CO2 – nonpolar; NOT soluble e) PH3 – polar molecule; YES dissolves in water 6. Which of the following will dissolve in C6H14 (hexane)? Hexane is a nonpolar solvent; therefore, nonpolar molecules will dissolve in this solvent a) SCl2 – polar molecule; NOT soluble in hexane b) O2 – nonpolar molecule; YES soluble in hexane c) NaCl – ionic compound; NOT soluble in hexane d) CO2 – nonpolar; YES soluble in hexane e) PH3 – polar molecule; NOT soluble in hexane Melting Point For a substance to melt, kinetic forces (increased by increasing temperature) must overcome the IMF’s keeping the particles in a fixed position. The stronger the intermolecular force, the higher the melting point. Remember that a freezing point has the same value as a melting point and can be determined exactly the same way. 7. Rank the following substances from highest melting point to lowest melting point: KNO3, H2O, N2O, NaCl, F2, Cl2 NaCl > KNO3 > H2O > N2O > Cl2 > F2 Determine the type of IMF in each molecule then use that information to arrange from high to low melting points: Electrostatic forces in the salts: KNO3 and NaCl. Smaller ions have stronger forces of attraction, so NaCl is smaller and therefore has a higher melting point. LDF in F2 and Cl2 lowest melting points. LDF’s stronger with larger molecule; therefore, Cl2 has a higher melting point than F2. Water hydrogen bonds. That makes it weaker than the salts but stronger than the halogens. N2O is a polar molecule with dipole-dipole forces. Boiling Point Boiling points are always higher than melting point for any one substance. The method for ranking different substances is the same as that for melting points: the stronger the IMF forces require higher temperature to escape the attraction and become a gas (boil). Ranking assumes constant atmospheric pressure. Lower atmospheric pressure decreases the b.p. of any substance. Boiling Points & Bond Types 8. Rank the following substance from highest boiling point to lowest boiling point: CaS, HNO3, KBr, SO2, XeF4 CaS> KBr > HNO3 > SO2 > XeF4 Determine the IMF’s to rank b.p.: CaS and KBr are ionic compounds electrostatic forces. CaS will have stronger forces because they have a higher charge of 2 than KBr with a charge of 1. HNO3 will hydrogen bond (H – N) SO2 is a polar covalent molecule; therefore, dipole-dipole bonds. XeF4 is a nonpolar covalent molecule; therefore, LDF. Vapor Pressure Vapor pressure is determine by the number of molecules that can escape from the surface of a liquid. More molecules can escape if the force of attraction is weak or if the force pulling them away (KE measured by temp) is high. Highest vapor pressure has the weakest IMF’s Temperature vs Vapor Pressure Curve 9. Rank the following substances from highest vapor pressure to lowest vapor pressure: Br2, NH3, Ar, PCl3, PCl5 Ar > Br2 > PCl5 > PCl3 > NH3 First look at type of IMF: NH3 hydrogen bonds Br2 and Ar have LDF because Br2 is nonpolar and Ar has no bonds. PCl3 is polar. PCl5 is nonpolar. Look at molar mass of nonpolar molecules: PCl5 (208.5 g/mol); Br2 (159.8 g/mol); and Ar (39.95 g/mol) Rank – highest vapor pressure has weakest IMF’s Viscosity and Surface Tension Depends on both temperature and IMF’s of the substance. Increase the temperature decreases both viscosity and surface tension. At any give temperature, the substance with stronger IMF’s has greater viscosity and higher surface tension. 10. Rank the following substance from highest vapor pressure to lowest vapor pressure: Br2, H2O, CCl4, PH3 H2O >PH3 > Br2, > CCl4 Liquids with stronger IMF’s have higher viscosity. 11. Rank the following substance from highest vapor pressure to lowest vapor pressure: Br2, H2O, CCl4, PH3 H2O >PH3 > Br2, > CCl4 Liquids with stronger IMF’s have higher surface tensions.