Document
advertisement
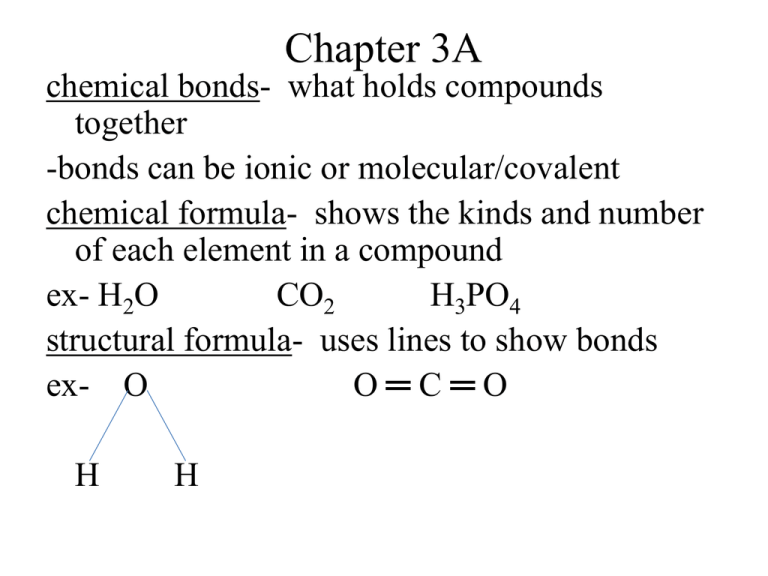
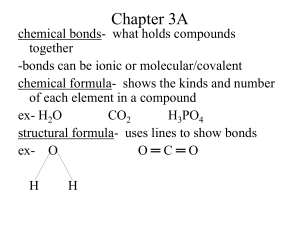
Chapter 3A chemical bonds- what holds compounds together -bonds can be ionic or molecular/covalent chemical formula- shows the kinds and number of each element in a compound ex- H2O CO2 H3PO4 structural formula- uses lines to show bonds ex- O O═C═O H H empirical formula- gives the relative number of atoms of each element in a compound -lowest whole number ratio molecular formula- gives the actual number of atoms of each element in a compound ex- hydrogen peroxide H2O2 emp. = HO (H and O in a 1:1 ratio) mol. = H2O2 page 87 Ex 3.1 and FP 3.1 atomic elements- exist in nature as single atoms -almost all the elements can exist as atoms -called monatomic molecular elements- cannot exist in nature as one atom, cannot exist alone -exist as molecules H O N Cℓ Br I F -called diatomic Ions- particles with a charge (+ or -), they have either lost or gained electrons -in ions # of p+ ≠ # of ecation -ion with a positive charge -loses electrons -metallic elements lose electrons -look at Group # to get the charge for Groups 1A to 3A ex- sodium Na1+ or Na+1 or Na+ aluminum Aℓ3+ -sodium lost 1e-, aluminum lost 3 e**Most cations of the transition metals have more than one charge -these will need to be given to you ex- Pb4+ lost 4e-a few have only one charge Ag1+ Zn2+ Cd2+ -silver lost 1e-, zinc and cadmium each lost 2e- anions -ion with a negative charge -gains electrons -non-metallic elements gain electrons -look at Group # - 8 to get the charge ex- chlorine Cℓ1arsenic As3-chlorine gained 1e-arsenic gained 3e*Group 4 elements do not generally form ions, Group 8 do not b/c they are inert Naming Ions Cations -name is the same as the element with the word ion -back to examples sodium ion aluminum ion lead (IV) ion Anions -drop ending and add –ide and the word ion -back to examples -chloride ion -arsenide ion Try these!! -name the ion/tell if it is a cation or anion -tell the charge on the ion -tell how e- the ion has lost or gained Sr I Ca K P B S polyatomic ions- made up or two or more atoms that carry a charge *most names end in –ite or –ate Ionic Compounds -compounds composed of cations and anions -made up of a metal (cation) and a non-metal (anion) -usually solid crystals at room temp -have high melting points -are electrically neutral because # of p+ = # of eex- NaCℓ KI Ca3N2 formula unit- chemical formula for an ionic compound Molecular Compounds -made up of two or more non-metals Binary Molecular Compounds -made up of two non-metals Ex- CO, CO2, CCℓ4 -to name molecular compounds you use prefixes Prefix monoditritetrapentahexaheptaoctanonadeca- # (subscript) 1 2 3 4 5 6 7 8 9 10 Naming Molecular Compounds -look at subscript of each element and give each element a prefix -if first element has a 1 as the subscript, then it does not get a prefix (omit mono-) -second element gets prefix and ends in –ide -if element begins with a vowel, drop the vowel at the end of a prefix **when writing formulas for molecular compounds you DO NOT reduce subscripts Try these!! carbon monoxide carbon tetrachloride sulfur trioxide tetriodine nonoxide phosphorus pentafluoride N2O CℓO8 PCℓ3 NF3 SF6 S2Cℓ2 OF2 N 2O 4 Acids -acids are compounds dissolved in water -will have (aq) after the formula which means aqueous or dissolved in water -always begin with hydrogen (H) Ex- HCℓ H2SO4 H3PO3 Naming Acids -you must look at what follows the hydrogen -if it is a single element (ends in –ide), then you use prefix hydro-, root of the element, -ic ending and the word acid ex- HCℓ hydrochloric acid -if what follows hydrogen ends in –ite, you just add –ous ending to root of the polyatomic ion and add acid ex- H3PO3 phosphorous acid -if what follows hydrogen ends in –ate, you just add –ic ending to root of the polyatomic ion and add acid ex- H2SO4 sulfuric acid Try these!! HI H2S H2CO3 H2SO3 HNO2 HCℓO3