Document

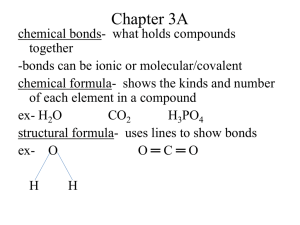
Chapter 3A
chemical bonds- what holds compounds together
-bonds can be ionic or molecular/covalent chemical formula- shows the kinds and number of each element in a compound ex- H
2
O CO
2
H
3
PO
4 structural formula- uses lines to show bonds exO O ═ C ═ O
H H
empirical formula- gives the relative number of atoms of each element in a compound
-lowest whole number ratio molecular formula- gives the actual number of atoms of each element in a compound ex- hydrogen peroxide
H
2
O
2 emp. = HO (H and O in a 1:1 ratio) mol. = H
2
O
2 page 87 Ex 3.1 and FP 3.1
atomic elements- exist in nature as single atoms
-almost all the elements can exist as atoms
-called monatomic molecular elements- cannot exist in nature as one atom, cannot exist alone
-exist as molecules
H O N Cℓ Br I F
-called diatomic
Ions- particles with a charge (+ or -), they have either lost or gained electrons
-in ions # of p + ≠
# of e cation
-ion with a positive charge
-loses electrons
-metallic elements lose electrons
-look at Group # to get the charge for Groups 1A to 3A
ex- sodium Na 1+ or Na +1 or Na + aluminum
Aℓ 3+
-sodium lost 1e-, aluminum lost 3 e -
**Most cations of the transition metals have more than one charge
-these will need to be given to you ex- Pb 4+ lost 4e -
-a few have only one charge
Ag 1+ Zn 2+ Cd 2+
-silver lost 1e-, zinc and cadmium each lost 2e -
anions
-ion with a negative charge
-gains electrons
-non-metallic elements gain electrons
-look at Group # - 8 to get the charge ex- chlorine
Cℓ 1arsenic As 3-
-chlorine gained 1e -
-arsenic gained 3e -
*Group 4 elements do not generally form ions,
Group 8 do not b/c they are inert
Naming Ions
Cations
-name is the same as the element with the word ion
-back to examples sodium ion
Anions aluminum ion lead (IV) ion
-drop ending and add –ide and the word ion
-back to examples
-chloride ion
-arsenide ion
Try these!!
-name the ion/tell if it is a cation or anion
-tell the charge on the ion
-tell how e the ion has lost or gained
Sr
P
I
B
Ca
S
K polyatomic ions- made up or two or more atoms that carry a charge
*most names end in –ite or –ate
Ionic Compounds
-compounds composed of cations and anions
-made up of a metal (cation) and a non-metal
(anion)
-usually solid crystals at room temp
-have high melting points
-are electrically neutral because # of p+ = # of e ex- NaCℓ KI Ca
3
N
2 formula unit- chemical formula for an ionic compound
Molecular Compounds
-made up of two or more non-metals
Binary Molecular Compounds
-made up of two non-metals
Ex- CO, CO
2
, CCℓ
4
-to name molecular compounds you use prefixes
Prefix monoditritetrapentahexaheptaoctanonadeca-
# (subscript)
1
2
5
6
3
4
7
8
9
10
Naming Molecular Compounds
-look at subscript of each element and give each element a prefix
-if first element has a 1 as the subscript, then it does not get a prefix (omit mono-)
-second element gets prefix and ends in –ide
-if element begins with a vowel, drop the vowel at the end of a prefix
**when writing formulas for molecular compounds you DO NOT reduce subscripts
Try these!!
carbon monoxide carbon tetrachloride sulfur trioxide tetriodine nonoxide phosphorus pentafluoride
N
2
O
CℓO
8
PCℓ
3
NF
3
SF
S
2
6
Cℓ
2
OF
N
2
2
O
4
Acids
-acids are compounds dissolved in water
-will have (aq) after the formula which means aqueous or dissolved in water
-always begin with hydrogen (H)
Ex- HCℓ H
2
SO
4
H
3
PO
3
Naming Acids
-you must look at what follows the hydrogen
-if it is a single element (ends in –ide), then you use prefix hydro-, root of the element, -ic ending and the word acid ex- HCℓ hydrochloric acid
-if what follows hydrogen ends in –ite, you just add –ous ending to root of the polyatomic ion and add acid ex- H
3
PO
3 phosphorous acid
-if what follows hydrogen ends in –ate, you just add –ic ending to root of the polyatomic ion and add acid ex- H
2
SO
4 sulfuric acid
Try these!!
HI
H
2
SO
3
H
2
S
HNO
2
H
2
CO
HCℓO
3
3
Chemical Equations reactants
products reactant- starting substance in a chemical reaction product- substance formed in a chemical reaction
= yields, gives you, produces, goes to
**Law of Conservation of Mass holds true here mass of reactants = mass of products
-the states of matter can be indicated after the substance
(s)=solid (ℓ)=liquid (g)=gas (aq)=aqueous
= reversible reaction catalyst- substance that speeds up the rate of a reaction without being used up in the reaction
**written above the arrow ex- Pt
∆ = heat applied
**Remember H O N Cℓ Br I F skeleton equation- chemical equation that is not balanced ex- Write a skeleton for the following reaction: solid iron reacts with oxygen to form solid iron
(III) oxide
Fe + O
2
Fe
2
O
3
Try these:
1) solid sodium hydrogen carbonate reacts with hydrochloric acid to produce aqueous sodium chloride, water and carbon dioxide
2) solid sulfur burns in oxygen to form sulfur dioxide
3) solid potassium chlorate forms oxygen and solid potassium chloride in the presence of catalyst manganese(II) oxide
Answers
1)NaHCO
3
(s) + HCℓ(aq)
NaCℓ(aq) + H
2
O (ℓ)
+ CO
2
(g)
2) S(s) + O
2
(g)
SO
2
(g)
3) KCℓO
3
(s)
MnO
O
2
(g) + KCℓ(s)
Balancing Equations
-each side of the equation (reactants and products) must have the same # of each element
-some may already be balanced
-must be lowest whole # ratio
-balance by putting coefficients in front of compounds
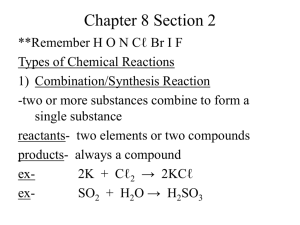
Types of Chemical Reactions
1) Combination/Synthesis Reaction
-two or more substances combine to form a single substance reactants- two elements or two compounds products- always a compound exex-
2K + Cℓ
2
SO
2
2KCℓ
+ H
2
O
H
2
SO
3
Try these!! Don’t forget to balance!!
a) aluminum + oxygen b) copper + sulfur (two possible reactions) c) beryllium + oxygen d) strontium + iodine e) magnesium + nitrogen a) 4Aℓ + 3O
2
2Aℓ
2
O
3 b) 2Cu + S
Cu
2
S or Cu + S
CuS c) 2Be + O
2 d) Sr + I
2
SrI
2 e) 3Mg + N
2
2BeO
Mg
3
N
2
2) Decomposition Reaction
-one compound breaks down or decomposes into two simpler compounds
-the reverse of synthesis ex- 2H
2
O
2H
2
+ O
2
Try these!!
a) lead(IV) oxide
b) hydrogen iodide
c) hydrogen bromide
d) sodium chloride
a) PbO
2
Pb + O
2 b) 2HI
H
2
+ I
2 c) 2HBr
H
2
+ Br
2 d) 2NaCℓ
2Na + Cℓ
2
3) Single-Replacement Reactions
-one element replaces a second element in a compound ex- 2K + CaO
K
2
O + Ca
-whether one metal will replace another metal is determined by reactivity of the metal
activity series of metals- lists metals in order of decreasing reactivity
ex- Mg + Zn(NO
3
)
2
*is Mg above Zn on the reactivity series?
Mg + Zn(NO
3
)
2
Mg(NO
3
)
2
+ Zn ex- Mg + Ag
2
Mg + Ag
2
SO
4
SO
4
MgSO
4
+ 2Ag ex- Mg + LiNO
3
-lithium is above magnesium
Mg + LiNO
3
no reaction
-Halogens can replace each other in singlereplacement reactions
-Reactivity decreases as you go down the halogen group
Try These!!
a) zinc + hydrogen sulfate
b) chlorine + sodium bromide
c) zinc + sodium nitrate
d) iron(II) + lead(II) nitrate
e) chlorine + sodium iodide
a) Zn + H
2 b) Cℓ
2
SO
4
H
2
+ 2NaBr
Br
2
+ ZnSO
4
+ 2NaCℓ c) Zn + NaNO
3
no reaction d) Fe + Pb(NO
3
)
2 e) Cℓ
2
+ 2NaI
I
2
Pb + Fe(NO
+ 2NaCℓ
3
)
2
4) Double-Replacement Reactions
-involve an exchange of cations between two reacting compounds ex- BaCℓ
2
+ K
2
CO
3
BaCO
3
+ 2KCℓ ex- FeS + 2HCℓ
H
2
S + FeCℓ
2
Try These!!
a) sodium hydroxide + iron(III) nitrate
b) barium nitrate + hydrogen phosphate
c) potassium hydroxide+hydrogen phosphate
d) hydrogen sulfate + aluminum hydroxide
Answers a) 3NaOH + Fe(NO
3
)
3 b) 3Ba(NO
3
)
2 c) 3KOH + H
3
+ 2H
3
PO
PO
4
4
Ba
3
(PO
4
)
2
K
3
PO
4
+ 3H
2
O
+ 6HNO
3 d) 3H
2
SO
4
+ 2Aℓ(OH)
3
3NaNO
3
+ Fe(OH)
3
Aℓ
2
(SO
4
)
3
+ 6H
2
O
5) Combustion Reaction
-hydrocarbon combined with oxygen to produce carbon dioxide and water ex- C
6
H
6
+ O
2
CO
2
+ H
2
O
-to balance:
C
C x
6
H
H y
6
+ O
2
CO
2
+ H
2
O
(x+y/4) (x) (y/2)
+ 7.5O
2
6CO
2
+ 3H
2
O
-multiply by 2 to get whole number ratio
2C
6
H
6
+ 15O
2
12CO
2
+ 6H
2
O
Try These!!
a) C
14
H
26 b) C
8
H
12
+ O
2
+ O
2
CO
2
CO
2
+ H
2
O
+ H
2
O
Answers!
a) 2C
14
H
26 b) C
8
H
12
+ 41O
2
+ 11O
2
28CO
2
8CO
2
+ 26H
+ 6H
2
O
2
O
Summary of Reactions
1) Combination/Synthesis
R + S
RS
2) Decomposition Reaction
RS
R + S
3) Single-Replacement Reaction
T + RS
R + TS
4) Double-Replacement Reaction
RS + TU
RU + TS
5) Combustion Reaction
C x
H y
+ O
2
CO
2
+ H
2
O