Nernst Presentation Notes
advertisement
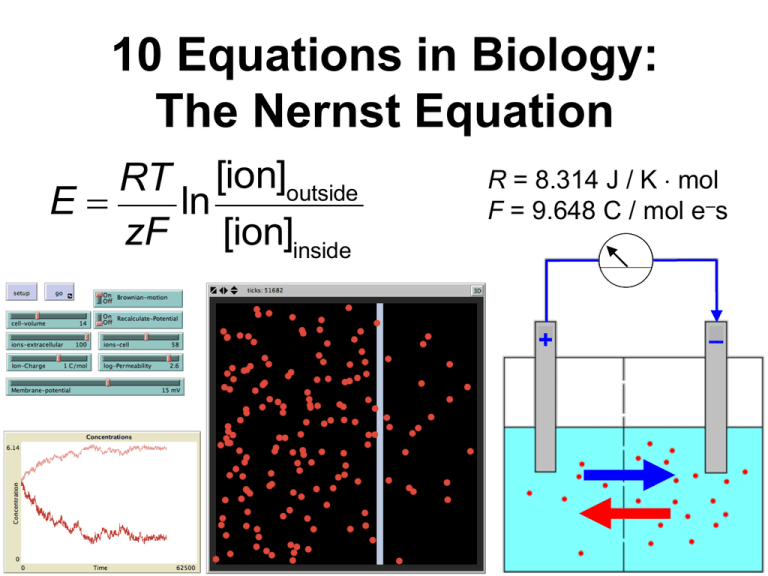
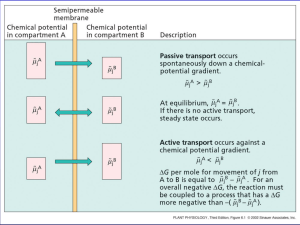
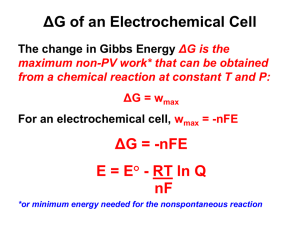
10 Equations in Biology: The Nernst Equation RT [ion]outside E= ln zF [ion]inside R = 8.314 J / K mol F = 9.648 C / mol e–s + – Focal Teaching Strategies 1. Informally derive an equation, step by step, from prior scientific knowledge. 2. Verbally deconstruct an equation, and interpret individual components. 3. Analyze an equation’s quantitative behavior, and translate the results into clear biological predictions. Intro: The Nernst Equation Determine the amount of elec. potential E needed across a membrane to prevent an ion from diffusing in response to its concentration gradient. Example • 2-compartment electrical cell • [Na+] higher on right, so net diffusion occurs toward the left. • Apply elec. current ERHS < 0: equilibrium b/n diffusion & electrostatic attraction e–s + – Background Knowledge What familiar scientific principles might be most relevant to understanding this system? • Individual ions have diff. charges. • Like charges repel; opposite charges attract. • Molecules diffuse down their conc. gradient. • There are certain universal physical constants. • Absolute zero temp. means no molecular movement. • others? 1. Informal Derivation • List 3-4 specific parameters that you would expect to influence a membrane’s equilibrium potential E for a particular ion. • For each parameter, would you expect it to have a positive or a negative effect on E? Parameter Effect on E (+ or –) 1. Informal Derivation • Now write an equation for E that includes all the parameters you listed, and in which each parameter has the effect you predicted. • Use dimensional analysis to verify that units are combined appropriately. E = T - d +... ? (degrees Kelvin) (micrometers) 2. Verbal Deconstruction RT [ion]outside E= ln zF [ion]inside • Dimensional analysis: units for E? • What do the constants R and F actually measure? Why are they present in the Nernst equation? 3. Mathematical Analysis & Biological Interpretation Compare & contrast the behavior of the actual Nernst equation with the variants shown below. Nernst: RT [ion]outside E= ln zF [ion]inside Why the log ratio? Variant 1: RT æ [ion]outside ö E= çç ÷÷ zF è [ion]inside ø Variant 2: RT E= [ion]outside - [ion]inside zF ( ) 3. Mathematical Analysis & Biological Interpretation RT [ion]outside E= ln zF [ion]inside • Under what conditions does the Nernst equation predict E = 0 ? Do those predictions make sense biologically? • What does the Nernst equation predict about the behavior of uncharged solutes? Is this prediction biologically reasonable? 3. Mathematical Analysis & Biological Interpretation • Assuming that the Nernst equation is an accurate model, predict the equilibrium potential for Na+ ions in a human skeletal muscle cell. RT [ion]outside E= ln zF [ion]inside • [Na+]cell 15 mM; [Na+]extracellular fluid 145 mM. • Intepret your result: effect of the membrane potential? how is it maintained by the cell? Take-home message • Informal derivation is faster than full derivation using word & formal equations, but less thorough. • Verbal deconstruction helps identify & remedy gaps in prior knowledge, and gives abstract concepts a more concrete foundation. • Mathematical analysis can be used to shift focus away from manipulating an equation to interpreting the underlying model and its outcomes.