View Poster - Idaho EPSCoR
advertisement
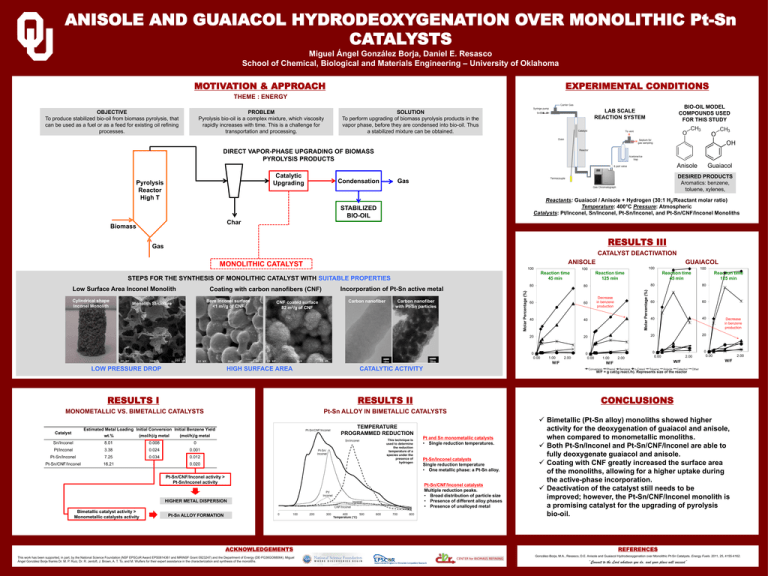
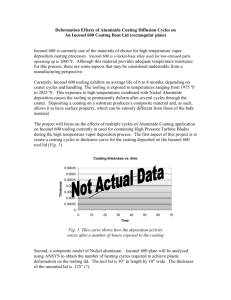
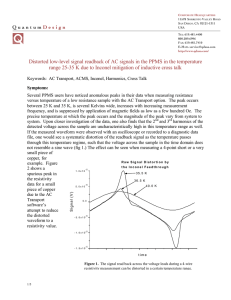
ANISOLE AND GUAIACOL HYDRODEOXYGENATION OVER MONOLITHIC Pt-Sn CATALYSTS Miguel Ángel González Borja, Daniel E. Resasco School of Chemical, Biological and Materials Engineering – University of Oklahoma MOTIVATION & APPROACH EXPERIMENTAL CONDITIONS THEME : ENERGY OBJECTIVE To produce stabilized bio-oil from biomass pyrolysis, that can be used as a fuel or as a feed for existing oil refining processes. PROBLEM Pyrolysis bio-oil is a complex mixture, which viscosity rapidly increases with time. This is a challenge for transportation and processing. BIO-OIL MODEL COMPOUNDS USED FOR THIS STUDY LAB SCALE REACTION SYSTEM SOLUTION To perform upgrading of biomass pyrolysis products in the vapor phase, before they are condensed into bio-oil. Thus a stabilized mixture can be obtained. DIRECT VAPOR-PHASE UPGRADING OF BIOMASS PYROLYSIS PRODUCTS Anisole Catalytic Upgrading Pyrolysis Reactor High T DESIRED PRODUCTS Aromatics: benzene, toluene, xylenes, Gas Condensation Guaiacol Reactants: Guaiacol / Anisole + Hydrogen (30:1 H2/Reactant molar ratio) Temperature: 400°C Pressure: Atmospheric Catalysts: Pt/Inconel, Sn/Inconel, Pt-Sn/Inconel, and Pt-Sn/CNF/Inconel Monoliths STABILIZED BIO-OIL Char Biomass RESULTS III Gas CATALYST DEACTIVATION ANISOLE 100 Reaction time 45 min Monolith Structure Bare Inconel surface <1 m2/g of CNF CNF coated surface 82 m2/g of CNF Incorporation of Pt-Sn active metal Carbon nanofiber Carbon nanofiber with Pt-Sn particles 80 Molar Percentage (%) Cylindrical shape Inconel Monolith Coating with carbon nanofibers (CNF) 60 60 40 40 20 20 1.00 W/F HIGH SURFACE AREA CATALYTIC ACTIVITY RESULTS II MONOMETALLIC VS. BIMETALLIC CATALYSTS Pt-Sn ALLOY IN BIMETALLIC CATALYSTS HIGHER METAL DISPERSION Bimetallic catalyst activity > Monometallic catalysts activity Pt-Sn ALLOY FORMATION ACKNOWLEDGEMENTS This work has been supported, in part, by the National Science Foundation (NSF EPSCoR Award EPS0814361 and MRINSF Grant 0923247) and the Department of Energy (DE-FG36GO88064). Miguel Ángel González Borja thanks Dr. M. P. Ruiz, Dr. R. Jentoft, J. Brown, A. T. To, and M. Wulfers for their expert assistance in the characterization and synthesis of the monoliths. Reaction time 45 min 0 0.00 1.00 2.00 Reaction time 125 min 80 80 60 60 40 40 20 20 0 0.00 2.00 W/F W/F Decrease in benzene production 0 0.00 2.00 W/F CONCLUSIONS TEMPERATURE PROGRAMMED REDUCTION Pt-Sn/CNF/Inconel activity > Pt-Sn/Inconel activity 2.00 Decrease in benzene production 100 W/F = g cat/(g react./h). Represents size of the reactor RESULTS I This technique is used to determine the reduction temperature of a species under the presence of hydrogen Reaction time 125 min 80 0 0.00 LOW PRESSURE DROP 100 100 STEPS FOR THE SYNTHESIS OF MONOLITHIC CATALYST WITH SUITABLE PROPERTIES Low Surface Area Inconel Monolith GUAIACOL Molar Percentage (%) MONOLITHIC CATALYST Pt and Sn monometallic catalysts • Single reduction temperatures. Pt-Sn/Inconel catalysts Single reduction temperature • One metallic phase: a Pt-Sn alloy. Pt-Sn/CNF/Inconel catalysts Multiple reduction peaks. • Broad distribution of particle size • Presence of different alloy phases • Presence of unalloyed metal Bimetallic (Pt-Sn alloy) monoliths showed higher activity for the deoxygenation of guaiacol and anisole, when compared to monometallic monoliths. Both Pt-Sn/Inconel and Pt-Sn/CNF/Inconel are able to fully deoxygenate guaiacol and anisole. Coating with CNF greatly increased the surface area of the monoliths, allowing for a higher uptake during the active-phase incorporation. Deactivation of the catalyst still needs to be improved; however, the Pt-Sn/CNF/Inconel monolith is a promising catalyst for the upgrading of pyrolysis bio-oil. REFERENCES González-Borja, M.A., Resasco, D.E. Anisole and Guaiacol Hydrodeoxygenation over Monolithic Pt-Sn Catalysts. Energy Fuels. 2011, 25, 4155-4162. “Commit to the Lord whatever you do, and your plans will succeed”