Ch03-acids-12-ques
advertisement

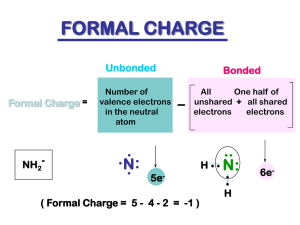
Chapter 3 Structure and Bonding Acids and Bases Acids & Bases/ Organic Chemistry Dr. Ron Rusay Fall 2012 Models of Acids and Bases •Arrhenius: Acids produce H+ & bases produce OH ion in aqueous solutions . •Brønsted-Lowry: Acids are H+ donors & bases are proton acceptors. •HCl + H2O Cl + H3O+ acid base Comprehensive Tutorial Acid & Base Principles in Organic Chemistry Highly recommended viewing http://chemconnections.org/organic/Movies%20Organic/acidsbases.mov Acid-Base Equilibrium Conjugate Acid/Base Pairs HA(aq) + H2O(l) H3O+(aq) + A(aq) acid base conj acid conj base •conjugate acid: formed when the proton is transferred to the base. •conjugate base: everything that remains of the acid molecule after a proton is lost. http://chemconnections.org/organic/Movies%20Org%20Flash/ ConjugateAcidBaseActivity.swf QUESTION: 3.1 Aniline, C6H5NH2, was isolated in the 1800s and began immediate use in the dye industry. What is the formula of the conjugate acid of this base? A. B. C. D. E. C6H5NH2+ C6H5NH3+ C6H5NH– C6H5NH+ C6H5NH2 Weak Acids • Weak acids are only partially ionized in solution. H3O+(aq) + A-(aq) HA(aq) + H2O(l) HA(aq) [H 3O ][A- ] Ka [HA] H+(aq) + A-(aq) or [H ][A- ] Ka [HA] • Ka is the acid dissociation constant. Organic Acids & Bases • Organic acids are weak acids, eg. Acetic acid. • However, there can be substantial differences in their relative strengths. What could you use to compare relative acidities? • Organic bases are weak bases and relate to ammonia. • However, there can be substantial differences in their relative strengths. What could you use to compare relative basicity? Conjugates Ka x Kb = ? What do pKa and pKb refer to? pKa + pKb = ? Remember: pH + pOH = pKw Strengths of Bases from pKa Values Values for pKb are not commonly found in the reference literature. Therefore, a comparison of relative basicity is made by using the pKa values of the corresponding conjugate acids. Which is the stronger acid? Which is the stronger base? QUESTION: 3.2 Use information on the table above to determine which of the following bases would have the weakest conjugate acid: OC6H5–; C2H3O2–; A. B. C. D. OC6H5– C 2 H 3 O2 – OCl– NH3 OCl–; NH3 QUESTION: 3.3 Use information on the table above to determine the order of increasing base strength for the following bases: OC6H5–; C2H3O2–; ClO2–; A. B. C. D. OC6H5– < NH3 < C2H3O2– < ClO2– C2H3O2– < ClO2– < NH3 < OC6H5– ClO2– < C2H3O2– < NH3 < OC6H5– NH3 < OC6H5– < C2H3O2– < ClO2– NH3 Acid and Base Equilibria The equilibrium favors the weaker of the acid vs. its conjugate acid or base vs. its conjugate. Weak wins! http://chemconnections.org/organic/Movies%20Org%20Flash/acid-base-eq.swf QUESTION: 3.4 Consider the following equilibria. Identify the weaker of the two: acid vs. its conjugate acid in each reaction. Which reactions favor formation of product? O O 1. 2. + NH3 CH3 C pKa=4.7 OH + NH3 OH 4. + NH4 pKa=9 O O O CH3 C 3. CH3 C pKa=36 HCl + CH3OH Kpa=15.5 HCl + CH3OH pKa-=–7 CH3 CCH + NH2 5. pKa =26 6. CH3 CH3 + pKa=50 CH3 COH2 + NH2 pKa=–6 A. All do: 1-6 B. 1, 4, 5 H–Cl–H + CH 3O pKa < –15 C. 2, 3, 6 Cl D. 1, 5 + CH3 OH2 pKa=–2.5 CH3 CC + E. None do. NH 3 Kap=36 NH2 CH3 CH2 + NH3 pKa=36 Worksheet 5: Acids & Bases http://chemconnections.org/organic/chem226/226assign-12.html#Worksheets Organic Acids & Bases • Organic molecules in context can be considered as behaving relatively as weak acids or weak bases. • Formal Charge is important in considering which. • Knowing the Formal Charge allows a prediction. • (+) positive atoms behave acid-like, (-) negative atoms behave base-like. • This can be used in predicting how molecules will react--- or don’t react, and the products of reactions. Formal Charge / Acids & Bases Electrophiles / Nucleophiles / Reactivity .. CH3 H3C : O: N CH3 CH3 H 3C N .. CH 3 H O: :: CH 3 .. N H3 C : : CH 3 : O: H3 C .. : O: CH3 :: CH3 C: H3C H3C .. H H3C O H H O H :: CH3 H :O .. .. CH2 C CH3 CH3 CH 3 H3C O: CH3 H3C : O: .. H3 C O : :: H3 C CH3 CH2 Worksheet 1 http://chemconnections.org/organic/chem226/226assign-12.html#Worksheets Structure and Acid-Base Properties Important factors that effect acidity in binary compounds, eg HCl (having only two elements): • Bond Length (shorter = stronger bonds; lower acidity) • Bond Strength (longer = weaker bonds; higher acidity: more dissociation, more protons [hydronium ions] in solution) • Bond Polarity (smaller e.n. differences favor higher acidities) Electronegativity, Charge Density, Bond Polarity and Acid-Base Properties Explain which is the stronger acid: HBr or HF. HBr is a stronger acid than HF. The greater the e.n. difference, the more polar the bond, the shorter and stronger the bond, and since it is harder to dissociate (break), the weaker the acid. Strength of Oxyacids (Three atoms: ternary vs. binary) Induction or a ”Push-Pull” electronic effect influences the proton, which weakens the oxygen-hydrogen bond. Chlorine (higher e.n.) pulls electrons more than Iodine (lower e.n.) QUESTION: 3.5 • Rank 1.0M solutions of HBrO, HIO and HClO in order of increasing acidity. HBrO , Ka = 2.1 x 10-8 HIO , Ka = 2.3 x 10-11 HClO , Ka = 3.0 x 10-8 A) HBrO < HIO < HClO B) HIO < HBrO < HClO C) HClO < HBrO < HIO D) HIO < HClO < HBrO Question: 3.6 True (A) / False (B) • HBrO4 is a weaker acid than HBrO2. • • • • HClO2 , Ka = 1.2 x 10-2 HBrO , Ka = 2.06 x 10-8 HIO , Ka = 2.3 x 10-11 HClO , Ka = 3.0 x 10-8 QUESTION: 3.7 • Rank 1.0M solutions of HBrO, HIO and HClO in order of increasing pH HBrO , Ka = 2.1 x 10-8 HIO , Ka = 2.3 x 10-11 HClO , Ka = 3.0 x 10-8 A) HBrO < HIO < HClO B) HIO < HBrO < HClO C) HClO < HBrO < HIO D) HIO < HClO < HBrO QUESTION: 3.8 • Use the concept of induction “push-pull”. Rank the following organic acids in order of decreasing acidity from most acidic to least. 1) Br-CH2COOH 2) I-CH2COOH • A) 2 > 1 > 3 B) 3 > 2 > 1 • C) 2 > 3 > 1 D) 1 > 2 > 3 3) CH3COOH QUESTION: 3.9 Rank the following acids in order of decreasing acidity. A) ClCH2COOH C) Cl3CCOOH 1) A > B > C > D 2) D > C > B > A B) Cl2CHCOOH D) CH3COOH 3) C > B > A > D 4) B > C > A > D Question: 3.10 True (A) / False (B) Trichloroacetic acid, Cl3CCOOH, is more acidic than trifluoroacetic acid, F3CCOOH. Comparing Acid-Base Strengths • Consider four possible factors (ARIO)* that affect the stabilization of a formal charge. The more effectively a conjugate base can stabilize its formal negative charge the weaker it is as a base. Conceptually, consider that it is done by spreading the negative charge, lowering electron density, and/or by reducing orbital size, which increases electron proximity and attraction to the positive nucleus. These factors make for a weaker base compared to a molecule that cannot affect the charge distribution as well. The corollary is that its parent acid is a stronger acid. ARIO: The type of Atom that has the formal charge. (A more electronegative atom is better at stabilizing negative charge.) Resonance (Delocalizing the negative charge makes for a weaker base.) Induction (“Push-Pull” electronic effects.) The type of Orbital where the charge resides: *Professor David Klein, U. of Chicago Approximate pKa Values of Functional Groups Professor William Blatchley, Keene State College Induction Substituent Effects: X-CH2COOH “Electron Donating”: Positive effect on pKa, (Weaker Acid) eg. (butyric vs. acetic acid) “Electron Withdrawing”: Negative effect on pKa, (Stronger Acid) eg. chlorine pKa acetic acid (X = H-) 4.75 butyric acid (X = CH3CH2-) 4.85 chloroacetic acid (X = Cl-) 2.87 4-chlorobutyric acid (X = ClCH2CH2-) 4.52 3-chlorobutyric acid (X = CH3CH2Cl-) 4.05 2-chlorobutyric acid (CH3CH2CHCl-COOH) 2.88 alkyl groups are weakly electron donating chlorines are electron withdrawing (Note: the proximity of chlorine has an important effect) Question: 3.11 Using ARIO principles, predict which of the protons in the following structure of acetylsalicylic acid is the most acidic. An Organic Base in Context Erythroxylon spp. • It is a very valuable plant. The leaves are chewed by indigenous tribes in the Andes to boost their energy. • It has been used as a psycho-therapeutic, an opthalmic anesthetic and was purportedly used in a popular beverage many years ago that is at the heart of a $20 billion corporation. • However, both its base and conjugate acid are currently controlled substances under U.S. Federal Regulations: Title 21 secs. 329.1 & 1308.12 (1987). • Can you name the beverage and the base? The beverage reportedly produced using the extract of leaves of Erythroxylon coca: The compound: cocaine, is an organic base: Merck Index, #2450, 11th ed.: Caution: May be habit forming…. Acid -Base Chemistry (Physical Properties) • m.p. 98 oC • b.p. (very volatile > 90 oC) Solubility: • Water: 1.67 x 10-3 g/mL • CHCl3: 1.43 g/mL • Ether: 0.29 g/mL What structural feature makes cocaine a base? What simple compound can you relate it to? “Regular” Cocaine Conjugate Acid of Cocaine (Physical Properties) • m.p. >195 oC Solubility: • Water: 2.5 g/mL • CHCl3: 0.08 g/mL • Ether: insoluble What accounts for the differences in solubilities of the base and conjugate acid? Comparison (Physical Properties) • • m.p. 98 oC b.p. (very volatile > 90 oC) Solubility: • Water: 1.67 x 10-3 g/mL • CHCl3: 1.43 g/mL • Ether: 0.29 g/mL Crack Cocaine: (Base) • m.p. >195 oC Solubility: • Water: 2.5 g/mL • CHCl3: 0.08 g/mL • Ether: insoluble Regular Cocaine (Conjugate Acid) http://en.wikipedia.org/wiki/Cocaine Curved Arrows in Organic Reactions • When bonds break and form, electrons move, and we can show their electron movements with curved arrows. • Consider the following generic acid/base reaction. • Each double-headed, curved arrow above shows the movement of two electrons. (A single headed arrow represents a single electron, as in Free Radical Reactions.) • Viewing all of the curved arrows represents the reaction MECHANISM, which accounts for where the electrons came from, how they moved, and where they wound up. Acid -Base Reactions Acid Base Reactions QUESTION: 3.12 Aniline, C6H5NH2, was isolated in the 1800s and began immediate use in the dye industry. What is the formula of the species present in a solution of aniline that is at a pH = 2? A. B. C. D. E. C6H5NH2+ C6H5NH3+ C6H5NH– C6H5NH+ C6H5NH2 Which form is favored? Acid or Conjugate Base? Solution effects: The pH of the surroundings (i.e. independent of the acid-conjugate base pH contribution) controls which form is favored, acid or conjugate base, and relates to the following equation. pH pK a log HA A • If the pH of the surroundings is < than a compound’s pKa, the compound will exist primarily in its acidic form. •If the pH of the surroundings is > than a compound’s pK a, the compound will exist primarily in its basic form. • NOTE: Remember that a buffer solution (surroundings) maintains a nearly constant pH within certain parameters. A weak acid RCO2H, pKa = 5.2 50% acid 50% base 99% acid 1% base 90% acid 10% base 10% acid 90% base 1% acid 99% base QUESTION: 3.13 Aspirin has a pKa of 3.5. In the stomach, pH ~ 2, what form of aspirin is favored? QUESTIONS from Worksheet: The pKa of a general anesthetic, sodium pentothal, is 7.4. If a patient is given sodium pentothal orally instead of iv, will it put the patient to sleep? What information is needed to answer this fundamental anesthesiology (“gas passer”) question? A drug has a pKa of 7.8 and is a known teratogen. If given iv to a pregnant woman whose blood pH is within normal levels, will this drug cross the placenta and affect the baby? What information is needed to answer this anesthesiology (“gas passer”) question? Lewis Acids and Bases • Lewis Acid: electron pair acceptor • Lewis Base: electron pair donor • Example: Al3+ + 6 O H H Al H 3+ O H 6 Question: 3.14 Select the chemical equation that depicts the correct movement of electrons in the reactionof ammonia with hydrogen chloride. A) B) C) D) Acid-Base Reactions Showing a reaction with arrows CH3 SCH3 + BF3 CH3 + H2O CH3 O + CH3 SH (CH3)2 S–BF3 CH3 OH2 CH3 OH + CH3S O OH CH3 CCH3 + H3O H2O + CH3CCH3 HCC HCC–CH2–O + H2C=O CH2 =CH2 + HCl I + CH3Br CH2 –CH3 + Cl ICH3 + Br Worksheet 5: Acids & Bases http://chemconnections.org/organic/chem226/226assign-12.html#Worksheets Draw products for the following acid/base reactions, and label the conjugate products. CH3 CH3 CH3 CH3 Acid-Base Reactions Predicting Products O + HCO3 CH 3C OH B(CH3 )3 + (CH3) 3N NH3 + OH H 3C + MgCl2 C O H 3C H2SO 4 + CH3OCH3 BF3 + O O + AlCl3 CH 3C OH Worksheet 5: Acids & Bases http://chemconnections.org/organic/chem226/226assign-12.html#Worksheets Predicting if reactions occur and the products if they do a. b. c, HO + Br–CH3 HO–Br + HO + Br–CH3 HO–CH3 + Br CH 3 H–CC + H2 C=O H–CC–CH 2-O H–CC + H2 C=O H–CC–O–CH2 NH2 + H–OCH3 H2N–OCH3 + NH2 + H–OCH3 H2N–H + H OCH 3 Worksheet 5: Acids & Bases http://chemconnections.org/organic/chem226/226assign-12.html#Worksheets Question: 3.15 Using pKa values given in the structure below, what is the product of the following reaction (NH4+/NH3, pKa = 9.3/ pKb = 4.7)? Consider that the reaction is with 1 mole of each reactant. A. B. C. D. “Solvation”: Solvent Effects • A large amount of solvent, which surrounds a substrate, can profoundly affect the substrate’s properties. • Consider the following solvent and ion–dipole attractions of two different bases, which are both stabilized by the interactions. • The tert-butoxide ion is sterically hindered, and is not as well solvated as the ethoxide ion. QUESTION: 3.16 Which has a lower pKa in water? A) tert-butanol or B) ethanol Choosing a Reaction Solvent An appropriate SOLVENT for an acid/base reaction: Should be able to surround the reactants and facilitate their collisions, increasing the rate of reaction. Should NOT itself react with the reactants. Because water can act as an acid or a base, it has a LEVELING EFFECT on strong acids and bases, which are common reactants or intermediates in many organic reactions: Acids stronger than H3O+ cannot be used in water. Bases stronger than OH- cannot be used in water. Water would not be an appropriate solvent for the following reaction. WHY? NaNH2 + H2O → NH3 + NaOH Question: 3.17 Which of the following solvents would be the best choice to use in the reaction: A) CH3COOH B) C) D)