Formal Charge, Lewis Diagrams & Molecular Structure
advertisement
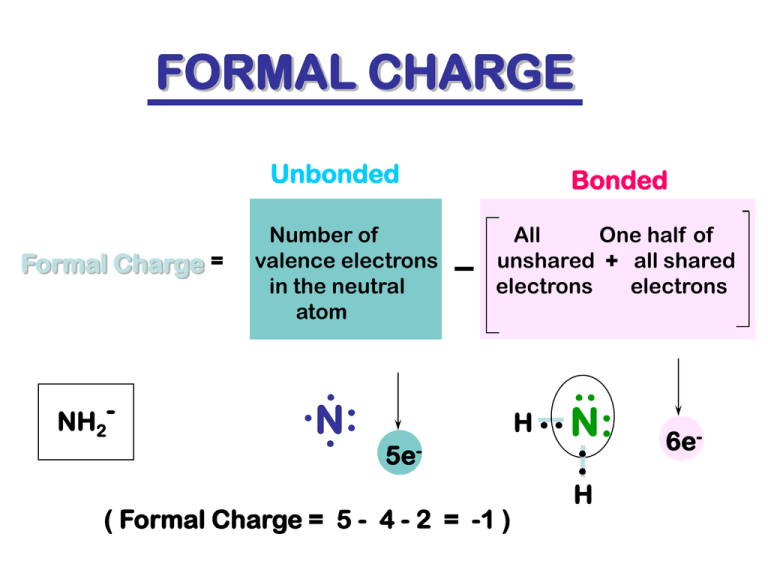
FORMAL CHARGE Unbonded Formal Charge = NH2- Number of valence electrons in the neutral atom . .N : . Bonded All One half of unshared + all shared electrons electrons 5e- ( Formal Charge = 5 - 4 - 2 = -1 ) .. H . . N: .. H 6e- When drawing a Lewis Diagram remember these rules. LEWIS DIAGRAMS SHOW IT ALL ! - all atoms including hydrogens - all bonds (lines not dots ) - all unshared pairs ( dots ) - all formal charges - all atoms with octets ( except H ) - the correct number of electrons ( count! ) Rumus Kimia Rumus empirik Rumus Molekul Rumus struktur Rumus struktur lengkap Rumus struktur panjang (expanded) Rumus struktur termampatkan (condensed) Rumus Struktur pada senyawa siklis – sikloheksana Expanded formula H H H H H H C C C C C C H H H H H H C 6 H 12 Rumus Struktur pada senyawa siklis – sikloheksana Polygon formula (condensed formula) C 6 H 12 Rumus Struktur pada senyawa siklis – sikloheksana Condensed formula CH 2 CH 2 CH 2 CH 2 CH 2 CH 2 C 6 H 12 Contoh Molekul siklis H H CH2 C H H C CH2 CH2 C H H C yc lo p ro p a n e H H H C 3H 6 H C C C C H H CH2 CH2 H C y clo b u ta n e H C 4H 8 H C H CH2 CH2 H CH2 H C C CH2 H CH2 H C H C H H CH2 CH2 H C y c lo p e n ta n e C 5H 10 Beberapa cara penulisan struktur c itro n e lla l (CH 3 ) 2 C=C HCH 2 CH 2 CH(CH 3 )CH 2 CH O or H 3C C CH CH 2 H 3C CH 2 CH 3 O C CH CH 2 H condensed H H H C O O H H C C H H C H H H H lin e s tru c tu re s a re m o s t c o m p a c t a n d e a s y to re a d H C H H C C C H C C H H H expanded lin e Molekul polar dan Nonpolar • To determine if a molecule is polar, we need to determine – if the molecule has polar bonds – the arrangement of these bonds in space • Molecular dipole moment (): the vector sum of the individual bond dipole moments in a molecule – reported in debyes (D) Bond Dipole Moments • are due to differences in electronegativity. • depend on the amount of charge and distance of separation. • In debyes, = 4.8 x (electron charge) x d(angstroms) Molecular Dipole Moments • Depend on bond polarity and bond angles. • Vector sum of the bond dipole moments. • Lone pairs of electrons contribute to the dipole moment. Polar and Nonpolar Molecules • these molecules have polar bonds, but each has a zero dipole moment Cl F O C B O F F Carbon dioxide =0D Boron trifluoride =0D C Cl Cl Cl Carbon tetrachloride =0D Polar and Nonpolar Molecules • these molecules have polar bonds and are polar molecules d i re cti o n of d i p ol e mo m ent N O H H H H H W at e r = 1 .8 5 D A m mo nia = 1.47D d i re cti o n of d i p ol e mo m ent Polar and Nonpolar Molecules – formaldehyde has polar bonds and is a polar molecule d i re cti o n of d i p ol e mo m ent O H C H Form al d e h y d e = 2.33 D Intermolecular Forces • Strength of attractions between molecules influence m.p., b.p., and solubility; esp. for solids and liquids. • Classification depends on structure. – Dipole-dipole interactions – London dispersions – Hydrogen bonding Dipole-Dipole => Dipole-Dipole Forces • Between polar molecules • Positive end of one molecule aligns with negative end of another molecule. • Lower energy than repulsions, so net force is attractive. • Larger dipoles cause higher boiling points and higher heats of vaporization. London Dispersions • • • • Between nonpolar molecules Temporary dipole-dipole interactions Larger atoms are more polarizable. Branching lowers b.p. because of decreased surface contact between molecules. CH3 CH3 CH3 CH2 CH2 CH2 n-pentane, b.p. = 36°C CH3 CH3 CH CH2 CH3 isopentane, b.p. = 28°C H 3C C CH3 CH3 neopentane, b.p. = 10°C => Dispersions => Hydrogen Bonding • Strong dipole-dipole attraction • Organic molecule must have N-H or O-H. • The hydrogen from one molecule is strongly attracted to a lone pair of electrons on the other molecule. • O-H more polar than N-H, so stronger hydrogen bonding H Bonds Boiling Points and Intermolecular Forces CH 3 CH 2 ethanol, b.p. = 78°C H 3C N O CH 3 dimethyl ether, b.p. = -25°C C H 3C H 2 CH3 N CH3 C H 3C H 2C H 2 trimethylamine, b.p. 3.5°C CH 2 ethylmethylamine, b.p. 37°C OH ethanol, b.p. = 78°C N H H H CH3 CH 3 CH 3 OH CH 3 propylamine, b.p. 49°C CH 2 NH 2 ethyl amine, b.p. 17°C ASAM DAN BASA Brønsted-Lowry Theory of Acids and Bases • Acid: • Base: Proton Donor Proton Acceptor Conjugate Acid: Base + Proton Conjugate Base: Acid - Proton Strong Acids and Bases • Strong acid - completely ionized in aqueous solution. Examples are: – HCl, HBr, HI, HNO3, HClO4, and H2SO4 • Strong base - completely ionized in aqueous solution. Examples are: – LiOH, NaOH, KOH, Ca(OH)2, and Ba(OH)2 Weak Acids and Bases • Acetic acid is a weak acid – it is incompletely ionized in aqueous solution O CH3 COH Acid (weaker acid) + H2 O Base (weaker base) O CH3 CO- Conjugate base of CH 3 CO 2 H (stronger base) + H3 O + Conjugate acid of H 2 O (stronger acid) Lewis Theory of Acids and Bases • Acid: Electron-Pair Acceptor – Electrophile • Base: Electron-Pair Donor – Nucleophile Weak Acids and Bases • The equation for the ionization of a weak acid, HA, in water and the acid ionization constant, Ka, for this equilibrium are HA + Ka = H2 O K eq [H pK a = - log K a A 2 O] = - [H + + 3 H3 O O ][A [HA] - ] + Weak Acids and Bases CH 3 CH 2 OH pK a 15.9 Conjugate Base CH 3 CH 2 O water H2 O 15.7 HO bicarbonate ion HCO 3 ammonium ion Acid Formula etha nol carbonic acid acetic acid - 2- 10.33 CO 3 NH 4 H 2 CO 3 9.24 NH 3 HCO 3 CH 3 CO 2 H 4.76 + 6.36 CH 3 CO 2 sulfuric acid H 2 SO 4 -5.2 HSO 4 hydrogen chloride HCl -7 Cl - - - Acidity Constant (Ka) K HA + H 2O A - - + + [A ] [H 3 O ] K = [H A ] [H 2 O ] - K [H 2 O ] = Ka = + [A ] [H 3 O ] [H A ] pKa = - lo g K a H 3O + pKa pKa = - log Ka Strong acid = large Ka = small pKa Weak acid = small Ka = large pKa Relative Acid Strength AC ID C O N J . B AS E s tro n g e r C lO 4 AC ID STRENGTH 4 10 10 -1 0 O O C H3 C pKa w eaker _ H C lO Ka C H3 C OH -5 1 .8 x 1 0 4 .8 O OH O B AS E STRENGTH -1 0 1 .0 x 1 0 10 _ C H3 C H3 w eaker -5 0 10 C H3 C H2 s tro n g e r 50 Acid Strength • Strong Acid – Conjugate base is weak – pKa is small • Weak Acid – Conjugate base is strong – pKa is large Base Strength • Strong Base – Conjugate acid is weak – pKa is large • Weak Base – Conjugate acid is strong – pKa is small Position of equilibrium • Favors reaction of the stronger acid and stronger base to give the weaker acid and weaker base + HA Stronger acid CH 3 CO 2 H Acetic acid pK a 4.76 (stronger acid) B - Stronger base + NH 3 A - + Weaker base CH 3 CO 2 - HB Weaker acid + NH + 4 Ammonium ion pK a 9.24 (weaker acid) Position of equilibrium • Stronger acid and stronger base react to give weaker acid and weaker base C H 3 C O2 H Acetic acid pK a 4.76 C 6 H 5 OH Phenol pK a 9.95 + + H C O3 - Bicarbonate ion H C O3 - Bicarbonate ion C H 3 C O2 Acetate ion C6 H5 O Phenoxide ion - - + H 2 C O3 Carbonic acid pK a 6.36 + H 2 C O3 Carbonic acid pK a 6.36