Theories of Acids and Bases - slider-dpchemistry-11
advertisement

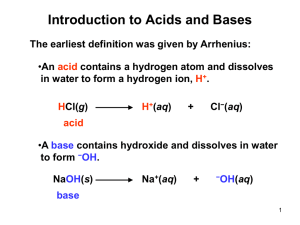
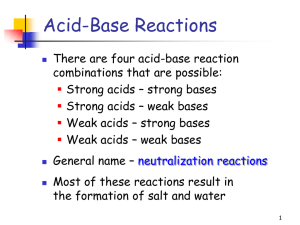
Year 12 Chemistry He classified all chemicals into three categories – acids, bases and salts He believed that all acids contained oxygen and it was this that gave them their sour taste Flaw: not all acids contain oxygen and metal oxides form bases Showed that all acids do not contain oxygen Proposed that acids are hydrogen containing materials following the discovery of HCl Flaw: not all substances that contain hydrogen are acids Acids dissociate in water forming H+ as one product Bases dissociate in water forming OHas one product Neutralisation involves the reaction of H+ and OH- forming a salt in water Flaws: theories only apply to aqueous solutions Some substances such as NH3 are bases and do not contain OH Relative strengths not addressed Amphoteric substances not addressed An acid is a proton (H+) donor A base is a proton acceptor Examples: HCl + H2O H3O+ + Cl¯ NH3 + H2O NH4+ + OH¯ Identify the acids/bases Any other acids/bases here? HCl + H2O H3O+ + Cl- NH3 + H2SO4 NH4+ + HSO4- HBr + NH2+ NH32+ + Br- Identify the B-L acids and bases in each of these reactions. Amphiprotic substances are those that can act as bases and acids. They can donate or accept protons. Water is an obvious example Notice in the previous slide that water reacts with both acids and bases. Bisulfate is another amphiprotic substance. Construct chemical equations to show this property. Lewis Theory An acid is an electron pair receptor A base is an electron pair donor Note that BF3 would not be an acid under the B-L Theory as there is no H+ to donate. A cobalt metal complex has 6 dative bonds formed by the donation of e- pairs from the ammonia molecules (Lewis bases) Which of these substances are Lewis bases? Lewis acids? Water reacts with carbon dioxide to form carbonic acid. Write out the structures and show how the electrons are transferred, thereby identifying the Lewis acid and Lewis base. Acid 1 + Base 2 Base 1 + Acid 2 Conjugate pair 1 Conjugate pair 2 What does this mean? An acid reacts and forms a conjugate base which can also accept a proton A base reacts and forms a conjugate acid which can donate a proton Conjugate pairs differ only by one H+ Example: HNO3 + H2O H3O+ + NO3¯ Identify the conjugate pairs in this reaction Here, nitric acid and the nitrate ion are conjugates and water and the hydronium ion are also conjugates Strong acids Weak acids completely dissociate in water Partially dissociate in water HA + H2O H3O+ + A- HA + H2O H3O+ + A- In general, these reactions are reversible, but for a strong acid the equilibrium is far right. So, There is an equilibrium established with weak acids, which means that there is less H3O+ ions in solution. HA + H2O H3O+ + A- CH3COOH + H2O H3O+ + CH3COO- Examples: HCl, HNO3, H2SO4 Examples: CH3COOH, H2CO3 What do you think is the difference in electrical conductivity? Strong bases Weak bases completely dissociate in water Partially dissociate in water NaOH Na+ + OH- Again, as with acids, the equilibrium is far right. So, 1 mol NaOH 1 mol OHExamples: group I hydroxides, Ba(OH)2 NH3 + H2O NH4+ + OHOnly about 1% of ammonia dissociates into hydroxide ions. 1 mol NH3 << 1mol OH- Examples: NH3, other amines Again, the electrical conductivity is greater for strong bases Predicting Equilibrium The direction of acid-base equilibria is away from the stronger acid base side and towards the weaker acid base side The stronger the acid, the weaker its conjugate base The stronger the base, the weaker its conjugate acid Reactions that proceed to a large extent: A strong acid will force the equilibrium in the opposite direction (in this case, forward or right) HCl + H2O H3O+ + Cl¯ Reactions that proceed to a small extent: If the weaker of the two acids and the weaker of the two bases are reactants (appear on the left side of the equation), the reaction is said to proceed to only a small extent: NH3 + H2O NH4+ + OH¯ Identify the conjugate acid base pairs in each reaction.