part 1 - Chemistry Courses
advertisement

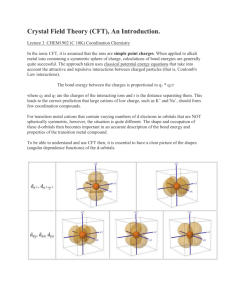
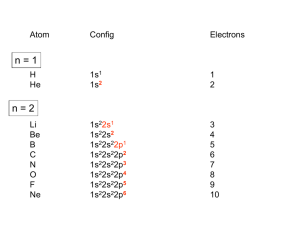
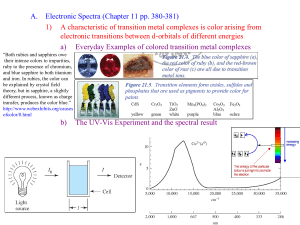
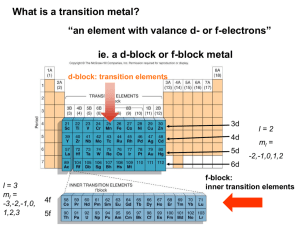
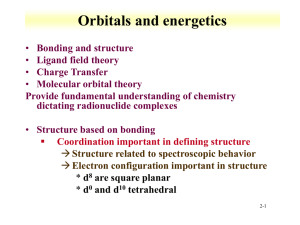
Coordination Chemistry – Ch. 5 Big-picture perspective: The interactions of the d orbitals with their surrounding chemical environment (ligands) influences their energy levels, and this coupled with the variable number of electrons (and incomplete filling of the d block) imparts on transition metals a unique, rich, and diverse chemistry. We will focus on developing and using a simple model for describing the bonding in octahedral transition metal complexes – a common molecular geometry – and then extend these concepts to non-octahedral complexes. This will allow us to describe many of the unique characteristics that are observed for transition metal complexes while setting the stage for more sophisticated models. Learning goals: • Determine oxidation states and assign d-electron counts for transition metals in complexes. • Derive the d-orbital splitting patterns for octahedral, elongated octahedral, square pyramidal, square planar, and tetrahedral complexes. • For octahedral and tetrahedral complexes, determine the number of unpaired electrons and calculate the crystal field stabilization energy. • Know the spectrochemical series, rationalize why different classes of ligands impact the crystal field splitting energy as they do, and use it to predict high vs. low spin complexes. Introduction Transition metals are central to life – biology, medicine, energy, technology, many things that society relies on to function The interactions of the d orbitals with their surrounding chemical environment (ligands) influences their energy levels, and this coupled with the variable number of electrons (and incomplete filling of the d block) imparts on transition metals a unique, rich, and diverse chemistry. Metal complexes We can describe metal complexes in several ways VERY important: Determining the d-electron count In order to predict their structures and understand their chemical reactivity and properties, we need to first consider the bonding in transition metal complexes and how the electrons are distributed Metal complex molecular orbitals As we have done for diatomic and triatomic molecules containing s- and pblock elements, one can generate a molecular orbital diagram by considering the overlap of ligand orbitals (s,p) with those on the transition metal (d). Crystal field theory We can simplify this process (go backwards in sophistication) by focusing exclusively on the d orbitals, since they contain the valence electrons. Crystal field theory is a simple way of describing the bonding in transition metal complexes. Here, we consider the metal ion acceptors (positive charge) in a “field” of the ligand electron pair donors (negative charge). Crystal field theory What is the origin of the different colors and magnetic properties of transition metal complexes? Crystal field theory Isolated ligand environment octahedral field [Fe(H2O)6]3+ Crystal field theory Let’s take a closer look at the splitting of d orbitals in an octahedral field eg orbitals L : L t2g orbitals : L dxy, dxz, dyz L dx2 - y2 dz 2 L L: :L L : : L high energy L low energy Crystal field theory Crystal field stabilization energy (CFSE) is the energy “gained” by putting valence electrons in the lower d-orbital set (t2g for octahedral complexes) Ti(H2O)63+ Cr(H2O)63+ Cu(H2O)62+ Crystal field theory What defines the magnitude of Δo? Crystal field theory Compare Δo across a set of metal complexes – what do these numbers mean? What are the colors of these compounds? Red light = 620 nm ≈ 16,000 cm-1 Blue light = 430 nm ≈ 23,000 cm-1 Crystal field theory 2nd and 3rd row transition metals (4d, 5d elements) always have larger values of Δo than 1st row transition metals (3d elements) – why? Crystal field theory Increasing oxidation state of the metal also increases Δo – why? Crystal field theory Ligands influence the magnitude of Δo – why? Crystal field theory What are the consequences of these multiple factors that influence the magnitude of Δo? Consider the following octahedral complexes – where do the electrons reside? Ti(H2O)63+ V(H2O)63+ Cr(H2O)63+ Mn(H2O)63+ Crystal field theory There is a small energy penalty associated with pairing electrons (electrostatic repulsion of electrons in the same orbital) For 3d elements, p (pairing energy) is approximately constant: ____________ How does the magnitude of p compare with typical magnitudes of Δo? What impact does this have on where electrons reside in Mn(H2O)63+? Crystal field theory Strong field ligands M : [Co(CN)6]4- Weak field ligands : [Co(H2O)6]2+ 4d and 5d transition metal complexes : high or low spin? Crystal field theory The spectrochemical series places ligands in order of increasing field strength, with a somewhat arbitrary cutoff between those we consider to be generally strong field ligands and those we consider to be generally weak field ligands. I– < < Br– py < < Cl– NH3 < < NO3– NO2– < < F– en < < OH– CN– How can we rationalize these trends? < < H2O CO Crystal field theory Some ligands are anomalously high in the spectrochemical series, considering their weak Lewis basicity. Why?