JonesSpr11
advertisement

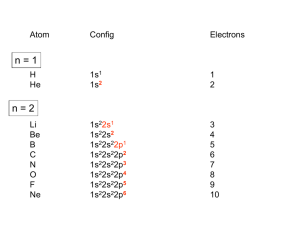
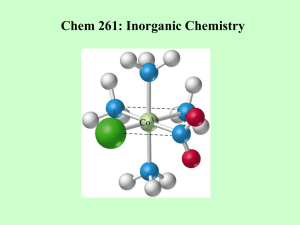
Synthesis, Structures and Ethylene Oligomerization Reactivity of Transition Metal Complexes Supported by Multidentate Amidine-Based Ligands † T. C. Jones, S. A. Bender, M. J. Carney, J. A. Halfen, B. L. Small , and O. L. Sydora † † Department of Chemistry, University of Wisconsin-Eau Claire, Eau Claire, Wisconsin 54702 and Chevron Phillips Chemical Company, 1862 Kingwood Drive, Kingwood, TX 77339 Goals and Objectives Multidentate Amidine-Based Ligands and Chromium Complexes: Synthesis and Structures Synthesize new families of multidentate ligands and exploit their ability to coordinate transition metals Develop new transition metal catalysts for olefin polymerization, including the oligomerization of ethylene to high purity a-olefins Develop new tridentate ligands to serve as analogues of Sasol-type catalysts Examine the impact of ligand structure (type, number and position of substituents) on catalyst performance Chromium Complexes Substituent Variation Catalyst performance attributes include the following: catalyst productivity, purity of a-olefins and overall a-olefin selectivity Background Information Tridentate ligands figure prominently in recent chromium catalyzed olefin polymerization studies. Cr-P 2.394 Å Cr-N(2) 2.011 Å Cr-O 2.077 Å Chromium pyridinebis(imine) complexes display high activities for ethylene oligomerization or polymerization, with polymer products being highly dependent on the ligand substituents. N(1) S N(2) N(1) Cl P Cl R' N N R' N Cr Cl MMAO Cl H H Me C 2H 4 R '' R'' H Me Me R''' t Bu H H P Product properties Cr N(3) N(2) Cr 1-butene, 99 % purity distribution of a-olefins polyethylene Cl Cl O Cr-P 2.424 Å Cr-N(2) 2.024 Å Cr-N(3) 2.237 Å Cl Cl O R ''' Small, Carney, et. al., Macromolecules 2004, 37, 4375-4386 Sasol has demonstrated that chromium complexes supported by tridentate PNP and SNS ligands display high activities for ethylene oligomerization, including the uncanny ability to selectively produce 1-hexene and 1-octene (the highest value a-olefins). H N N(2) Cl Cr Cl PR2 or RS Cr Cl Cl Cl PNP SNS SR 1 -h e x e n e a n d /o r 1 -o cte n e C 2H 4 N(3) Cl McGuinness, et. al., J. Am. Chem. Soc. 2003, 125, 5272 Cl N(2) P Transition metal complexes were isolated as greenish-blue to royal blue solids. Slow recrystallization provided samples suitable for x-ray analysis. Polymerizations (MMAO co-catalyst) were performed in a 500 mL autoclave in using the reactor conditions indicated in the table. Oligomeric products were analyzed by GC using the polymerization solvent as an internal standard. X-Ray crystallography and NMR spectroscopy of ligands and metal complexes confirm successful synthesis. Cl Cl Cr-P 2.438 Å Cr-N(2) 2.010 Å Cr-N(3) 2.143 Å Cl Cr-P 2.423 Å Cr-N(2) 2.129 Å Cr-N(3) 2.068 Å R R R T ( C) Activity (g/g cat-hr) CH2CH2NC4H8O Ph Ph 60 0 C6 fraction % a-olefin nd CH2CH2PPh2 Ph Ph 60 0 nd CH2CH2NMe2 Ph Ph 60 0 nd CH2CH2NMe2 Ph Ph 90 1000 92.9 C6H4-o-SPh Ph Ph Ph 90 10400 98.0 p-MeC6H4 Ph 60 4100 28.2 2,6-Me2C6H4 p-MeC6H4 Ph 60 75100 96.2 2,6-Me2C6H4 p-MeC6H4 i Pr 60 108000 99.3 2,6-Me2C6H4 p-MeC6H4 i Pr 90 301200 99.4 2,6-Me2C6H4 p-t BuC6H4 i 60 481000 99.5 1 2 3 Pr o Surprisingly, chromium complexes supported by tridentate ligands (Sasol analogues) are not active polymerization catalysts. However, chromium complexes supported by bidentate N-phosphino amidine ligands yield catalysts with high activity and product purity. Selected Polymerization Data Experimental Details N-phosphino amidine ligands were isolated as solids or viscous oils and characterized by 1H and 13C NMR spectroscopy. P N(3) McGuinness, et. al., J. Am. Chem. Soc. 2004, 126, 14712 Amidine ligand precursors were purified by crystallization or vacuum distillation and characterized by 1H and 13C NMR spectroscopy. Ligand synthetic methods are modular and versatile, allowing for a large array of substituent combinations. Cr Cr MMAO Cl N(1) N(1) H N Cl R 2P Conclusions and Future Work Polymerizations w ere performed for 30 minutes at 900 psi (850 psi ethylene, 50 psi hydrogen) in cyclohexane using MMAO as cocatalyst (Al:Cr = 1000) Continuing Work: Examine other ligand substituents and their effect on catalyst performance Prepare other transition metal (V, Fe, Ni, Pd, etc.) complexes using Nphosphino amidine ligands and examine their catalytic performance Conduct theoretical studies (DFT computations/molecular modeling) to help predict the impact of ligand modifications on metal complex geometry and catalytic behavior Acknowledgements The authors thank the UW-Eau Claire Office of Research and Sponsored Programs (ORSP) and Chevron Phillips Chemical Company for their generous financial support of this work.