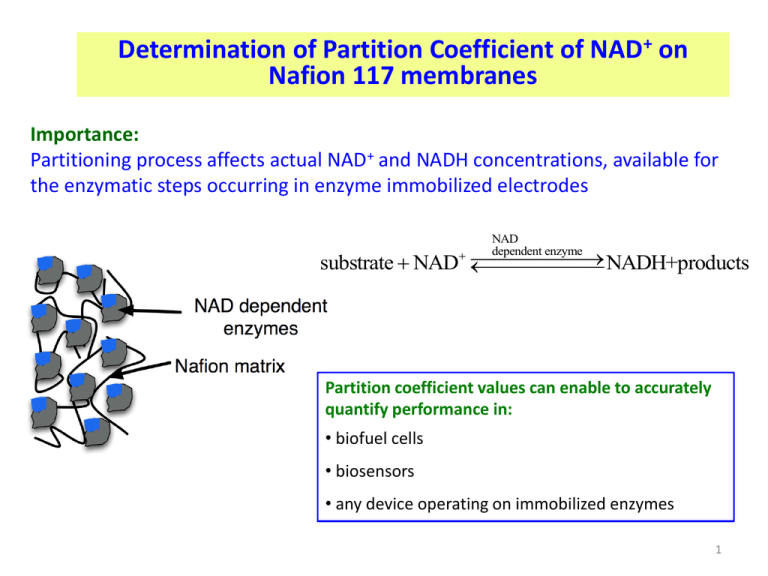
Determination of Partition Coefficient of NAD+ on
Nafion 117 membranes
Importance:
Partitioning process affects actual NAD+ and NADH concentrations, available for
the enzymatic steps occurring in enzyme immobilized electrodes
NAD
dependent enzyme
NADH+products
substrate NAD
Partition coefficient values can enable to accurately
quantify performance in:
• biofuel cells
• biosensors
• any device operating on immobilized enzymes
1
Determination of Partition Coefficient of NAD+ on
Nafion 117 membranes
Objective:
Determine NAD+ partition coefficient
Experimental parameters (current study):
• pH
• NaNO3 concentration
• NAD+ concentration
• Nafion types (hydrogen form and
sodium form)
Molecular structure
of Nafion (hydrogen form)
A perfluorinated sulfonic acid polymer2
Experimental Procedure
• Nafion 117 membrane, cut into a desired
size
• Preparation of External solution:
NAD+ and NaNO3, dissolved in a 10 mM
phosphate buffer solution at a desired pH
7.5
• Equilibration:
Nafion 117 membranes, soaked in the NAD+
solutions for at least 20 hours, to allow
electrochemical equilibrium between
membrane and external solution
• [NAD+] and pH measurements:
[NAD+] in Nafion membranes and external
solutions were measured by UV-Vis
0.1 mM [NAD+] gives an approximate
absorbance of approximately
3
16900 M-1 cm-1 at 260 nm
Equilibrium achieved within 22
hours (Nafion, hydrogen form)
UV-Vis spectra of Nafion after immersion
in NAD+ containing 0.8 M NaNO3 solution
for 22 and 96 hours
[NAD+] partitioning decreased
by addition of NaNO3 in
external solution
UV-Vis spectra of Nafion after immersion
in NAD+ containing 0.5 M NaNO3 solution
for 22 hours
4
EDX Line-scan Analysis for Sodium
a. 0.80 M NaNO3 in external solution
Left:
Scanning electron micrographs
of the cross-sections of the dry
Nafion 117 membranes after
partition coefficient study.
Scanned lines are shown.
b. 0.06 M NaNO3 in external solution
Right:
EDX along the scanned lines
showing sodium content [Na+]
c. no NaNO3 in external solution
Observation:
[Na+]in c. ≤ [Na+]in b. ≤ [Na+]in a.
Sodium content in Nafion
increased with content of
NaNO3 in external solution
5
Partition Coefficients at different
[NaNO3] in external solution
Partition coefficient, K,
is the slope of the
linear fit of plot of [NAD+]
in Nafion membrane
(as-received) to that inside
the external solution
As – received Nafion
and equilibrium pH 2.25
K ≈ 13 (absence of
NaNO3)
K ≈ 2 (as NaNO3
approaches 0.8 M)
6
Partition Coefficient at pH 7.0
Experiments conducted with deprotonated Nafion (A and B)
Nafion, A
- Immersed in In 1 M NaNO3 solution
at 75 °C for 18 hours
- Rinsing in DI water
Nafion, B
-Immersed in 1 M NaNO3 solution at
75 °C for 18 hours
De-protonated Nafion and equilibrium pH 7
Partition Coefficient (K) ≈ 0.05
(no NaNO3 in solution)
- heated in DI water at 75 °C for
6 hours
Implication: Low NAD+ partition coefficients make the sodium form of Nafion 117 membrane
unsuitable for enzyme immobilization
7
Donan Membrane Equilibrium Eq.
e
i,ext
RT ln(ai,ext ) zi Fext
e
i,Naf
RT ln(ai,Naf ) zi FNaf
ai,ext
ai,Naf
[
According to Donan Eq. condition:
e
e
i,ext
i,Naf
Electro-neutrality is conserved in each
compartment (membrane and external
solution)
[Donan, F. G. Journal of American
Chemical Society 1924, 1, 73 – 90]
exp[
ai,ext
ai,Naf
1
zi
zi F
(Naf ext )]
RT
] exp[
F
(Naf ext )] K
RT
Requirements:
• Two aqueous compartments separated
by a membrane (permeable to water
and ions)
• Fixed charge in the membrane
Applicability range ph < ≈ 4, because NAD+
remains charge neutral above that pH
[Moore Jr., C. E.; Underwood, A. L. Anal.
8
Biochem. 1969, 29, 149-153]
Calculation of Partition Coefficient (K)
At species concentrations much
lower than than the fixed charge
concentrations can be replace
activities, in order to apply the
Donan Membrane Equilibrium Eq.
Applying Donan membrane equilibrium eq.
for cationic species:
CNAD , Naf 1/1 CNa , Naf 1/1 CH , Naf 1/1
K [
] [
] [
]
CNAD , ext
CNa , ext
CH , ext
ai Ci
Applying Donan membrane equilibrium eq.
for anionic species:
K [
CNO , Naf
3
CNO , ext
] [
1/1
3
CH PO , Naf
2
4
CH PO , ext
2
] [
1/1
4
CHPO2 , Naf
4
CHPO2 , ext
] [
1/2
4
COH , Naf
COH , ext
I --- ion exchange capacity = 1.77 M
[L. A. Zook and J. Leddy, Analytical
Chemistry, 1996, 68, 3793 – 3797]
]1/1
K is determined by applying equilibrium membrane charge balance:
K{CNa , ext CNAD , ext CH , ext } I
(1 / K){CNO , ext CH PO , ext COH , ext } (1 / K 2 )CHPO2 , ext
3
2
4
4
9
Comparison: Calculated Partition Coefficients vs. those
measured at various pH
pH:
Equilibrium pH = 2.25
(measured) for external solution
Inside the Nafion membrane,
pH ≈ 1 or lower
(value not measured) and
assumed
Observations:
Calculated values, assuming unity
activity coefficients match
Measured partition coefficient
value
10
Main Conclusions
Equilibrium [NAD+] in Nafion 117 membranes was observed to be already
attained at 22 hours
Calculated partition coefficient (assuming unity activity coefficient) values
match with those measured at low pH.
Summary:
pH
Nafion type
[NaNO3]
NAD+ partition
coefficient
2.25
Hydrogen form
none
15
2.25
Hydrogen form
0.8
3
7
Sodium form
none
0.05
Ongoing and Future Work
Extend the study to other candidate materials for enzyme immobilization,
e.g. chitosan and Tetra Butyl Ammonium Bromide (TBAB) Nafion
Determine partition coefficient of NADH
11
NAD+ Activity Coefficient and pH inside Nafion membrane
Activity coeff. of NAD+
NAD is given by:
log( NAD ) 1.173Z 2
Is
1 Is
NAD
Is ci Zi2
i
Is is the ionic strength
inside Nafion
Z = +1, charge on NAD+
0.275 = < NAD > = 0.40
12