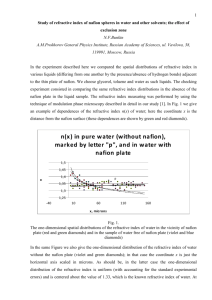
Presentation (PDF)
advertisement

Nafion: Hydration, Microstructure and Schroeder’s paradox Viatcheslav Freger Maria Bass , Amir Berman (BGU) Oleg Konovalov, Amarjeet Singh (ESRF) Technion – Israel Institute of Technology Wolfson Department of Chemical Engineering Haifa, Israel Nafion and Its Uses An ionomer developed by DuPont in 70s Fuel Cells Catalysis Sensors Membrane electrolysis Unique Microstructure: Microphase separation and 2D Micelle Morphology Hsu and Gierke, JMS, 1983 Schmidt-Rohr and Chen, Nat Mater., 2008 Gebel, Polymer, 2000 Gebel, Diat et al, Macromolecules, 2002, 2004 2D Morphology: Transport vs. Hydration Conductivity Water self-diffusion (NMR) 1 D/Dw 0.1 0.01 Blum et al. SPE PEEKK Nafion 2D 3D 0.001 0.01 0.1 water volume fraction XV Kreuer, JMS, 2001 VF et al., JMS, 1999 1 Schroeder’s Paradox: Two Isotherms? Sample Osmotic stressor solution Sample 30 vapor liquid l 20 Li-Nafion 10 0 0 0.2 0.4 0.6 water activity 0.8 1 Bass and Freger, 2008 Schroeder’s Paradox and Water Transport DwC w Jw w J H RT ~ 1 5 If the thermodynamic potential of water is ill-defined, how does one model water transport and “water management”? Schroeder’s paradox explained ? Choi and Datta (JES, 2003) were first to publish an explanation, but they assumed permanent pores; hydrophobic pore walls (despite ionic groups); stability of surface structure and 3-phase line. Fixing the Model: Structure and Equilibrium Four terms are the minimal set f go ( ) Gef ( 0 ) 2 BR / osmotic “inflation” interface “corona” R (ve v g ) 2 1 Minimize g = f – l to get (l) 4 3 5 VF, Polymer, 2003; JPC B, 2009 Chemical Equilibrium as Balance of Pressures out in d s s (1 )2 R R R 2/3 Pressures: g out , in - osmotic d - inflation (transient) s - interfacial-elastic (“Laplace”) The interfacial tension is zero, but the “Laplace” pressure is not unless = 1. l’ l” ’ ” VF, JPC B, 2009 Surface Equilibrium Two more equilibrium conditions at the surface: Balance of 3 tensions (Neumann construction) Equilibrium between polymer bulk and surface a 2 12 1 liquid (1) b matrix (2) vapor an ionic group c d e VF, JPC B, 2009 Surface Equilibrium: Interim Summary In vapor water gets buried under surface; s ≥ 0. In liquid micelles are inverted and s = 0 (Schroeder’s paradox). Nafion should dissolve in water, but dissolution never happens (relaxation time ≥ 105 s). However, (quasi-)dissolution may occur at the surface. normal-type micelles (“spaghetti”) surface-aligned bundle (“macaroni”) water s (1 ) 2 R Examining the Surface Structure: GISAXS d p ~ 3 nm c 0.2 for 8 keV Rubatat and Diat, Macrmolecules, 2007 (bulk SANS) ESRF and ID10B Nafion Surface in Vapor (GISAXS) 1 Qxy *I, A-1 a.u. 100 nm thick Nafion film spin-cast on a Si wafer T = 30 C, RH ~ 97% Beam 8 keV 0.11 0.17 0.2 0.25 0.1 0.01 0.001 0.01 0.1 Qxy , A-1 1 Bass et al., JPC B, 2010 10 GISAXS: Going Under Water Nafion film C18-capped Si substrate water vapor Vapor vs. Liquid: Contact Angle and AFM CA: Nafion surface is hydrophobic in vapor and hydrophilic in water AFM: under water the surface gets rougher (surface tension drops). Vapor RH=97% q = 94.5 ± 1.1 hydrophobic Air Air bubble Liquid water q = 25.4 ± 0.25 hydrophilic water Dry q = 96.4 ± 1.2 hydrophobic Air Water drop Water drop Hydrophilic vs. Hydrophobic Substrate Nafion film Nafion film Native Si substrate (SiO2) C18-capped Si substrate OTS on Si: z = -59 mV, q = 130o (Yang & Abbott, Langmuir, 2010) Dura et al., Macromolecules, 2009 (NR) Micelle Orientation at Interfaces Water bundles breaking up Vapor Nafion film C18-capped Si substrate Bass et al., 2010 a micelle bundle Micelle bundles Native Si (SiO2) substrate Some of these are metastable nonequilibrium structures! (non-relaxed elastic stress, relaxation time >105 s) Balsara et al, NanoLett, 2007 Summary Solid Nafion is a non-equilibrium structure. Non-relaxed pressures in Nafion result in a non-thermodynamic behavior (Schroeder’s paradox); this needs to be accounted for in transport modeling. Interfaces affect the morphology and orientation of micelles in thin Nafion films; this could be attractive for developing barriers with enhanced and stable transport characteristics. Vapor Nafion Liquid s (1 ) 2 R Thanks ISF ESRF Maria Bass Oleg Konovalov, Amarjeet Singh, Jiři Novak (ESRF, ID10B) Amir Berman, Yair Kaufman, Juergen Jopp (BGU) Special thanks: Emmanuel Korngold (BGU), Klaus-Dieter Kreuer, Martin Ise (MPI Stuttgart) Another old puzzle: microscopic vs. macroscopic swelling The relative change of Bragg spacing (d-do)/d (“microscopic swelling”) may be compared with the relative macroscopic linear expansion (1/p – 1)1/3 calculated from l. Though for high l the relation is as for dilute 2D micelles, for solid Nafion (small and moderate l) it is nearly linear, as if the structure is 1D (lamellae) Gebel, 2000; Fujimura et al., 1981, 1982 Microscopic vs. macroscopic swelling The model shows a good agreement with scattering data, provided a 2D morphology is “plugged in” Microscopic swelling 6 100 D=3 5 D=2 4 D=2 var dmax, nm 3 10 2 constant variable 1 1 0 0 0.5 1 1.5 0.01 0.1 p Linear expansion n 1 D d d0 1 1 g 1 1 D p 1 n w d0 D g D g n 2 3 for D=2 theoretical initial slope is 7 (exp 6) 1