Benzene and Its Derivatives Ch#4
advertisement

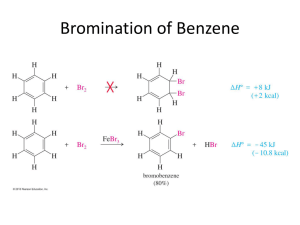
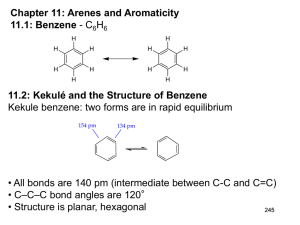
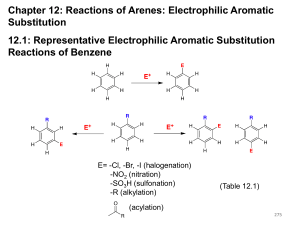
Aromatic Compounds Discovery of Benzene • Isolated in 1825 by Michael Faraday who determined C:H ratio to be 1:1. • Synthesized in 1834 by Eilhard Mitscherlich who determined molecular formula to be C6H6. • Other related compounds with low C:H ratios had a pleasant smell, so they were classified as aromatic. Benzene Structures • Proposed in 1866 by Friedrich Kekulé, shortly after multiple bonds were suggested. • Failed to explain existence of only one isomer of 1,2-dichlorobenzene. H H C C H C C C H C H H Kekule Structure Prismane Structure Benzene Structures • Each sp2 hybridized C in the ring has an unhybridized p orbital perpendicular to the ring which overlaps around the ring. Resonance Structure => Unusual Reactions • Alkene + KMnO4 diol (addition) Benzene + KMnO4 no reaction. • Alkene + Br2/CCl4 dibromide (addition) Benzene + Br2/CCl4 no reaction. • With FeCl3 catalyst, Br2 reacts with benzene to form bromobenzene + HBr (substitution!). Double bonds remain. Unusual Stability Annulenes • All cyclic conjugated hydrocarbons were proposed to be aromatic. • However, cyclobutadiene is so reactive that it dimerizes before it can be isolated. • And cyclooctatetraene adds Br2 readily. [6]anulene [4]anulene [8]anulene What Does It Take to Be Aromatic? • Alternating double and single bonds • A magic number of pi electrons • Resonance structures must be able to move the pi electrons in a circular manar. • Non bonding electrons can also participate in the resonance structures. Hückel’s Rule • Hückel’s Rule is used to generate the magic number of pi electrons. • If the compound has a continuous ring of overlapping p orbitals and has 4N + 2 pi electrons, it is aromatic. • If the compound has a continuous ring of overlapping p orbitals and has 4N electrons, it is antiaromatic. • N is any integer, starting at zero Generation of Magic Pi Electrons Value of N 0 1 2 3 4N + 2 2 6 10 14 [N]Annulenes • [4]Annulene is antiaromatic (4N e-’s) • [8]Annulene would be antiaromatic, but it’s not planar, so it’s nonaromatic. • [10]Annulene is aromatic except for the isomers that are not planar. • Larger 4N annulenes are not antiaromatic because they are flexible enough to become nonplanar. Tropylium Ion • The cycloheptatrienyl cation has 6 p electrons and an empty p orbital. • Aromatic: more stable than open chain ion. H H OH + H , H2O + Which of the Following are Aromatic? a. e. b. f. c. g. d. h. Disubstituted Benzenes • The prefixes ortho-, meta-, and para- are • commonly used for the 1,2-, 1,3-, and 1,4• positions, respectively. => 3 or More Substituents • Use the smallest possible numbers, but • the carbon with a functional group is #1. OH O 2N NO2 NO2 1,3,5-t rinit robenzene O 2N NO2 NO2 2,4,6-t rinit rophenol Common Names of Benzene Derivatives OH CH3 phenol toluene H C CH2 styrene OCH3 NH2 aniline anisole O O O C C C acetophenone CH3 benzaldehyde H benzoic acid OH Phenyl and Benzyl • Phenyl indicates the benzene ring • attachment. The benzyl group has • an additional carbon. Br phenyl bromide CH2Br benzyl bromide Fused Ring Hydrocarbons • Naphthalene • Anthracene • Phenanthrene Larger Polynuclear Aromatic Hydrocarbons • Formed in combustion (tobacco smoke). • Many are carcinogenic. • Epoxides form, combine with DNA base. pyrene => Physical Properties • Melting points: More symmetrical than corresponding alkane, pack better into crystals, so higher melting points. • Boiling points: Dependent on dipole moment, so ortho > meta > para, for disubstituted benzenes. • Density: More dense than nonaromatics, less dense than water. • Solubility: Generally insoluble in water. Electrophilic Aromatic Substitution Electrophile substitutes for a hydrogen on the benzene ring. Chapter 17 21 Mechanism Step 1: Attack on the electrophile forms the sigma complex. Step 2: Loss of a proton gives the substitution product. Chapter 17 22 Bromination of Benzene • Requires a stronger electrophile than Br2. • Use a strong Lewis acid catalyst, FeBr3. Br Br FeBr3 Br H Br H Br - FeBr3 H H H + H + Br FeBr3 H H Br + H _ + FeBr4 H H H Br + Chapter 17 HBr 23 Comparison with Alkenes • Cyclohexene adds Br2, H = -121 kJ • Addition to benzene is endothermic, not normally seen. • Substitution of Br for H retains aromaticity, H = -45 kJ. • Formation of sigma complex is rate-limiting. Chapter 17 24 Energy Diagram for Bromination Chapter 17 25 Chlorination and Iodination • Chlorination is similar to bromination. Use AlCl3 as the Lewis acid catalyst. • Iodination requires an acidic oxidizing agent, like nitric acid, which oxidizes the iodine to an iodonium ion. + H + + HNO 3 + 1/2 I2 I Chapter 17 + NO 2 + H2O 26 Nitration of Benzene Use sulfuric acid with nitric acid to form the nitronium ion electrophile. O H O S O H O H O H O N H O N + O O O H O H O N + O O H2O + N+ _ + HSO4 NO2+ then forms a sigma complex with benzene, loses H+ to form nitrobenzene. O Chapter 17 27 Sulfonation Sulfur trioxide, SO3, in fuming sulfuric acid is the electrophile. O _ O O S S+ O O O O O S+ _ O O _ O S O O H O S O + O H _ S + O O HO O S O benzenesulfonic acid Chapter 9 28 Nitration of Toluene • Toluene reacts 25 times faster than benzene. The methyl group is an activating group. • The product mix contains mostly ortho and para substituted molecules. Chapter 17 29 Sigma Complex Intermediate is more stable if nitration occurs at the ortho or para position. => Chapter 17 30 Energy Diagram Chapter 17 31 Activating, O-, P-Directing Substituents • Alkyl groups stabilize the sigma complex by induction, donating electron density through the sigma bond. • Substituents with a lone pair of electrons stabilize the sigma complex by resonance. OCH3 + OCH3 NO2 NO2 + H H Chapter 17 32 Substitution on Anisole Chapter 17 33 The Amino Group Aniline, like anisole, reacts with bromine water (without a catalyst) to yield the tribromide. Sodium bicarbonate is added to neutralize the HBr that’s also formed. Chapter 17 34 Summary of Activators Chapter 17 35 Deactivating Meta-Directing Substituents • Electrophilic substitution reactions for nitrobenzene are 100,000 times slower than for benzene. • The product mix contains mostly the meta isomer, only small amounts of the ortho and para isomers. • Meta-directors deactivate all positions on the ring, but the meta position is less deactivated. Chapter 17 36 Ortho Substitutionon Nitrobenzene Chapter 17 37 Para Substitution on Nitrobenzene Chapter 17 38 Meta Substitution on Nitrobenzene Chapter 17 39 Energy Diagram Chapter 17 40 Structure of Meta-Directing Deactivators • The atom attached to the aromatic ring will have a partial positive charge. • Electron density is withdrawn inductively along the sigma bond, so the ring is less electron-rich than benzene. Chapter 17 41 Summary of Deactivators Chapter 17 42 More Deactivators Chapter 17 43 Halobenzenes • Halogens are deactivating toward electrophilic substitution, but are ortho, para-directing! • Since halogens are very electronegative, they withdraw electron density from the ring inductively along the sigma bond. • But halogens have lone pairs of electrons that can stabilize the sigma complex by resonance. Chapter 17 44 Sigma Complex for Bromobenzene Ortho and para attacks produce a bromonium ion and other resonance structures. No bromonium ion possible with meta attack. Chapter 17 45 Energy Diagram Chapter 17 46 Summary of Directing Effects Chapter 17 47 Multiple Substituents The most strongly activating substituent will determine the position of the next substitution. May have mixtures. OCH3 OCH3 SO3H SO3 O2N H2SO4 OCH3 + O2N O2N SO3H Chapter 17 48 Friedel-Crafts Alkylation • Synthesis of alkyl benzenes from alkyl halides and a Lewis acid, usually AlCl3. • Reactions of alkyl halide with Lewis acid produces a carbocation which is the electrophile. • Other sources of carbocations: alkenes + HF, or alcohols + BF3. Chapter 17 49 Examples ofCarbocation Formation Cl CH3 H2C OH H3C CH CH3 CH CH3 + AlCl3 HF CH CH3 BF3 _ CH3 + C Cl AlCl3 H3C H _ F + H3C CH CH3 + BF3 H O H3C CH CH3 Chapter 17 _ + H3C CH CH3 + HOBF3 50 Formation of Alkyl Benzene CH3 +C H H + CH3 H F H + CH(CH3)2 F CH(CH3)2 - B OH CH3 F CH + CH3 H Chapter 17 HF F B OH F 51 Friedel-CraftsAcylation • Acyl chloride is used in place of alkyl chloride. • The acylium ion intermediate is resonance stabilized and does not rearrange like a carbocation. • The product is a phenyl ketone that is less reactive than benzene. Chapter 17 52 Mechanism of Acylation O O C C+ R + H R Cl _ AlCl3 O C HCl R + AlCl3 H Chapter 17 53 Catalytic Hydrogenation • Elevated heat and pressure are required. • Possible catalysts: Pt, Pd, Ni, Ru, Rh. • Reduction cannot be stopped at an intermediate stage. CH3 CH3 3 H2, 1000 psi Ru, 100°C CH3 Chapter 17 CH3 54 Side-Chain Oxidation Alkylbenzenes are oxidized to benzoic acid by hot KMnO4 or Na2Cr2O7/H2SO4. CH(CH3)2 - KMnO4, OH CH CH2 H2O, heat Chapter 17 _ COO _ COO 55 Side-Chain Halogenation • Benzylic position is the most reactive. • Chlorination is not as selective as bromination, results in mixtures. • Br2 reacts only at the benzylic position. Br CH2CH2CH3 Br2, h Chapter 17 CHCH2CH3 56 AROMATIC REVIEW Give the shape of benzene. a. Tetrahedral b.Bent c. Trigonal pyramidal d.Planar Answer a. Tetrahedral b.Bent c. Trigonal pyramidal d.Planar All six carbons and six hydrogens are in the same plane. Give the hybridization of each carbon in benzene. a. b. c. d. sp sp2 sp3 sp4 Answer a. b. c. d. sp sp2 sp3 sp4 Each carbon in benzene is sp2 hybridized. Give the bond angle of the atoms in benzene. a. 45° b.60° c. 90° d.109.5° e.120° Answer a. 45° b.60° c. 90° d.109.5° e.120° The carbons are 120o apart in benzene. Classify a. Aromatic b.Antiaromatic c. Nonaromatic d.Acyclic . Answer a. Aromatic b.Antiaromatic c. Nonaromatic d.Acyclic The compound gives a whole number for N in Hűckel’s rule (4N + 2 = 6, N = 1). Classify a. Aromatic b.Antiaromatic c. Nonaromatic d.Acyclic . Answer a. Aromatic b.Antiaromatic c. Nonaromatic d.Acyclic The compound is cyclic and has continuous delocalized electrons, but does not give a whole number for Hűckel’s rule (4N + 2 = 8, N = 3/2). Classify a. Aromatic b.Antiaromatic c. Nonaromatic d.Acyclic . Answer a. Aromatic b.Antiaromatic c. Nonaromatic d.Acyclic This cyclic compound does not have a continuous, overlapping ring of p orbitals and is nonaromatic. Name a. Anthracene b.Naphthalene c. Phenanthrene d.Benzene Answer a. Anthracene b.Naphthalene c. Phenanthrene d.Benzene Naphthalene contains two benzene rings fused together. Name a. Anthracene b.Naphthalene c. Phenanthrene d.Benzene Answer a. Anthracene b.Naphthalene c. Phenanthrene d.Benzene Anthracene contains three benzene rings fused together. Identify how carbon, diamond, and graphite are related. a. They are enantiomers of carbon. b.They are diastereomers of carbon. c. They are allotropes of carbon. d.They have the same properties. Answer a. They are enantiomers of carbon. b.They are diastereomers of carbon. c. They are allotropes of carbon. d.They have the same properties. Name Cl NH2 Br a. 4-Bromo-3chloroaniline b.4-Bromo-3chlorophenol c. 4-Bromo-3chloroanisole d.1-Bromo-2-chloro-4aniline e.1-Bromo-2-chloro-4phenol Answer a. 4-Bromo-3chloroaniline b.4-Bromo-3chlorophenol c. 4-Bromo-3chloroanisole d.1-Bromo-2-chloro-4aniline e.1-Bromo-2-chloro-4phenol Aniline is the parent compound. The NH2 is at position one. CH3 Name OH a. b. c. d. e. p-Methylphenol m-Methylphenol o-Methylphenol 4-Methylphenol 3-Methylphenol Answer a. b. c. d. e. p-Methylphenol m-Methylphenol o-Methylphenol 4-Methylphenol 3-Methylphenol The groups are on adjacent carbons, which is ortho. Name O C NO2 H a. 3-Amino-5benzaldehyde b.5-Amino-3benzaldehyde c. 3-Aminobenzaldehyde d.5-Nitro-3benzaldehyde e.3-Nitrobenzaldehyde Answer a. 3-Amino-5benzaldehyde b.5-Amino-3benzaldehyde c. 3-Aminobenzaldehyde d.5-Nitro-3benzaldehyde e.3-Nitrobenzaldehyde Benzaldehyde is the parent compound. Name O C NO2 H a. o-Aminobenzaldehyde b. m-Aminobenzaldehyde c. p-Aminobenzaldehyde d. o-Nitrobenzaldehyde e. m-Nitrobenzaldehyde Answer a. o-Aminobenzaldehyde b. m-Aminobenzaldehyde c. p-Aminobenzaldehyde d. o-Nitrobenzaldehyde e. m-Nitrobenzaldehyde Benzaldehyde is the parent compound. Name C6H5CH2CH2C≡CCH3. a. 1-Phenyl-3-pentyne b.5-Phenyl-2-pentyne c. 4-Phenyl-2-pentyne d.1-Phenyl-2-butyne e.1-Phenyl-3-butyne Answer a. 1-Phenyl-3-pentyne b.5-Phenyl-2-pentyne c. 4-Phenyl-2-pentyne d.1-Phenyl-2-butyne e.1-Phenyl-3-butyne Identify the slow step in electrophilic aromatic substitution. a. Formation of a stronger nucleophile. b.Formation of the benzenonium ion. c. Deprotonation to regain aromaticity. d.Formation of a carbanion. Answer a. Formation of a stronger nucleophile. b.Formation of the benzenonium ion. c. Deprotonation to regain aromaticity. d.Formation of a carbanion. Benzene attacking the electrophile to form the benzenonium ion is the slow step. Cl2 AlCl3 a. Hexachlorobenzene b.Hexachlorocyclohexane c. 5,6-Dichloro-1,3-cyclohexadiene d.Chlorobenzene Answer a. Hexachlorobenzene b.Hexachlorocyclohexane c. 5,6-Dichloro-1,3-cyclohexadiene d.Chlorobenzene One chlorine atom substitutes on the benzene. 1. HNO3, H2SO4 2. Zn, aq. HCl a. Nitrobenzene b.Aniline c. Chlorobenzene d.Benzenesulfonic acid Answer a. Nitrobenzene b.Aniline c. Chlorobenzene d.Benzenesulfonic acid A nitro group is added, which is then reduced to an amino group. SO3 a. Nitrobenzene b.Aniline c. Chlorobenzene d.Benzenesulfonic acid Answer a. Nitrobenzene b.Aniline c. Chlorobenzene d.Benzenesulfonic acid A sulfonic acid group is added to the benzene. CH2CH3 HNO3 H2SO4 a. 2- and 4-nitroethylbenzene b.3-Nitroethylbenzene c. 2- and 4-ethylbenzenesulfonic acid d.3-Ethylbenzenesulfonic acid Answer a. 2- and 4-nitroethylbenzene b.3-Nitroethylbenzene c. 2- and 4-ethylbenzenesulfonic acid d.3-Ethylbenzenesulfonic acid The ethyl group is an ortho and para director. Classify a bromide. a. Meta, activating group b.Meta, deactivating group c. Ortho and para, deactivating group d.Ortho and para, activating group Answer a. Meta, activating group b.Meta, deactivating group c. Ortho and para, deactivating group d.Ortho and para, activating group The electrons can delocalize into the bromide, making another benzenonium ion intermediate. Classify a nitro group. a. Meta, activating group b.Meta, deactivating group c. Ortho and para, deactivating group d.Ortho and para, activating group Answer a. Meta, activating group b.Meta, deactivating group c. Ortho and para, deactivating group d.Ortho and para, activating group OCH3 AlCl3 CH3CH2CH2Cl a. 3-Propylanisole b.2- and 4-propylanisole c. 3-Isopropylanisole d.2- and 4-isopropylanisole Answer a. 3-Propylanisole b.2- and 4-propylanisole c. 3-Isopropylanisole d.2- and 4-isopropylanisole The methoxy group is an ortho, para director. The propyl group rearranges. Give the intermediate in a Friedel– Crafts acylation. a. Carbocation b.Carbanion c. Radical d.Acylium ion Answer a. Carbocation b.Carbanion c. Radical d.Acylium ion An R–C≡O+ is an intermediate in a Friedel–Crafts acylation. CO, HCl AlCl3, CuCl a. Chlorobenzene b.Benzoic acid c. Benzaldehyde d.Benzene Answer a. Chlorobenzene b.Benzoic acid c. Benzaldehyde d.Benzene The Gatterman–Koch formylation forms benzaldehyde. CH2CH2CH3 Br2 light a. 2-Bromopropylbenzene b.3-Bromopropylbenzene c. 4-Bromopropylbenzene d. -Bromopropylbenzene e. -Bromopropylbenzene Answer a. 2-Bromopropylbenzene b.3-Bromopropylbenzene c. 4-Bromopropylbenzene d. -Bromopropylbenzene e. -Bromopropylbenzene Bromine substitutes on the benzylic carbon. OH (CH3)2CHOH HF a. 2-Isopropylphenol b.3-Isopropylphenol c. 4-Isopropylphenol d.2-Isopropylphenol and 4-isopropylphenol e.2-Isopropylphenol and 3-isopropylphenol Answer a. 2-Isopropylphenol b.3-Isopropylphenol c. 4-Isopropylphenol d.2-Isopropylphenol and 4-isopropylphenol e.2-Isopropylphenol and 3-isopropylphenol Phenols are highly reactive substrates for electrophilic aromatic substitution. End of Chapter 9 Chapter 17 110