Abbreviated Chapter 17 Powerpoint
advertisement
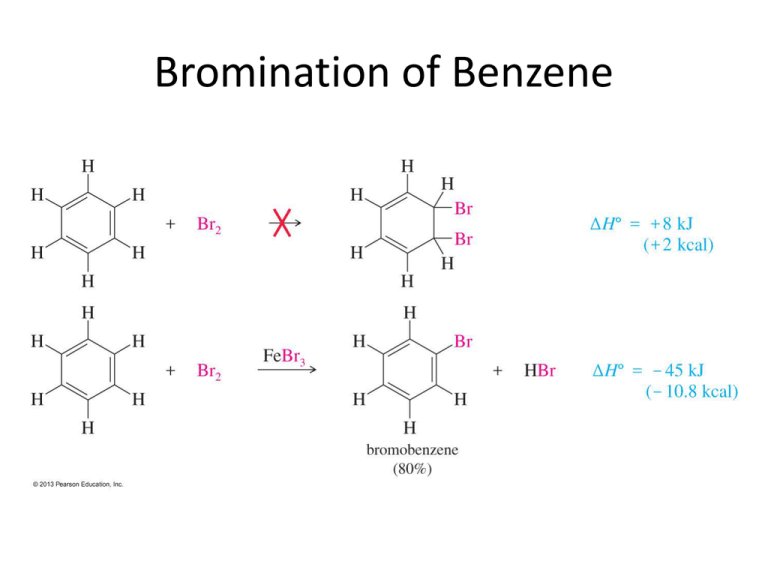
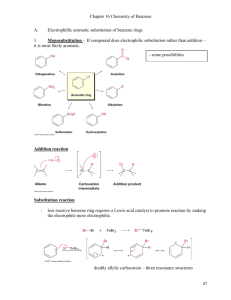
Bromination of Benzene Mechanism for the Bromination of Benzene: Preliminary Step • Before the electrophilic aromatic substitution can take place, the electrophile must be activated. • A strong Lewis acid catalyst, such as FeBr3, should be used. Mechanism for the Bromination of Benzene: Steps 1 and 2 Step 1: Electrophilic attack and formation of the sigma complex. Step 2: Loss of a proton to give the products. Chlorination of Benzene • Chlorination is similar to bromination. AlCl3 is most often used as catalyst, but FeCl3 will also work. Nitration of Benzene • Sulfuric acid acts as a catalyst, allowing the reaction to be faster and at lower temperatures. • HNO3 and H2SO4 react together to form the electrophile of the reaction: nitronium ion (NO2+). Mechanism for the Nitration of Benzene: Preliminary Step • Formation of the nitronium ion is the preliminary step of the reaction. Mechanism for the EAS Nitration of Benzene Step 1: Formation of the sigma complex. Step 2: Loss of a proton gives nitrobenzene. Friedel–Crafts Alkylation • Synthesis of alkyl benzenes from alkyl halides and a Lewis acid, usually AlCl3. • Reactions of alkyl halide with Lewis acid produces a carbocation, which is the electrophile. Mechanism of the Friedel–Crafts Reaction Step 1 Step 2 Step 3 Rearrangements Protonation of Alkenes • An alkene can be protonated by HF. • This weak acid is preferred because the fluoride ion is a weak nucleophile and will not attack the carbocation. Alcohols and Lewis Acids • Alcohols can be treated with BF3 to form the carbocation. Limitations of Friedel–Crafts • Reaction fails if benzene has a substituent that is more deactivating than halogens. • Rearrangements are possible. • The alkylbenzene product is more reactive than benzene, so polyalkylation occurs. Friedel–Crafts Acylation • Acyl chloride is used in place of alkyl chloride. • The product is a phenyl ketone that is less reactive than benzene. Mechanism of Acylation Step 1: Formation of the acylium ion. Step 2: Electrophilic attack to form the sigma complex. Mechanism of Acylation (Continued) Step 3: Loss of a proton to form the product. The Gattermann-Koch Reaction Friedel–Crafts acylations are generally free from rearrangements and multiple substitution. They do not go on strongly deactivated rings, however. Sulfonation of Benzene • Sulfur trioxide (SO3) is the electrophile in the reaction. • A 7% mixture of SO3 and H2SO4 is commonly referred to as “fuming sulfuric acid.” • The —SO3H group is called a sulfonic acid. Sulfur Trioxide • Sulfur trioxide is a strong electrophile, with three sulfonyl bonds drawing electron density away from the sulfur atom. Desulfonation Reaction • Sulfonation is reversible. • The sulfonic acid group may be removed from an aromatic ring by heating in dilute sulfuric acid. Nitration of Toluene • Toluene reacts 25 times faster than benzene. • The methyl group is an activator. • The product mix contains mostly ortho and para substituted molecules. Ortho and Para Substitution • Ortho and para attacks are preferred because their resonance structures include one tertiary carbocation. Energy Diagram Meta Substitution • When substitution occurs at the meta position, the positive charge is not delocalized onto the tertiary carbon, and the methyl group has a smaller effect on the stability of the sigma complex. Alkyl Group Stabilization • Alkyl groups are activating substituents and ortho, paradirectors. • This effect is called the inductive effect because alkyl groups can donate electron density to the ring through the sigma bond, making them more active. Anisole • Anisole undergoes nitration about 10,000 times faster than benzene and about 400 times faster than toluene. • This result seems curious because oxygen is a strongly electronegative group, yet it donates electron density to stabilize the transition state and the sigma complex. Substituents with Nonbonding Electrons Resonance stabilization is provided by a pi bond between the —OCH3 substituent and the ring. Meta Attack on Anisole • Resonance forms show that the methoxy group cannot stabilize the sigma complex in the meta substitution. Bromination of Anisole • A methoxy group is so strongly activating that anisole is quickly tribrominated without a catalyst. Summary of Activators Activators and Deactivators • If the substituent on the ring is electron donating, the ortho and para positions will be activated. • If the group is electron withdrawing, the ortho and para positions will be deactivated. Nitration of Nitrobenzene • Electrophilic substitution reactions for nitrobenzene are 100,000 times slower than for benzene. • The product mix contains mostly the meta isomer, and only small amounts of the ortho and para isomers. Ortho Substitution of Nitrobenzene • The nitro group is a strongly deactivating group when considering its resonance forms. The nitrogen always has a formal positive charge. • Ortho or para addition will create an especially unstable intermediate. Meta Substitution on Nitrobenzene • Meta substitution will not put the positive charge on the same carbon that bears the nitro group. Energy Diagram Deactivators and Meta-Directors • Most electron-withdrawing groups are deactivators and meta-directors. • The atom attached to the aromatic ring has a positive or partial positive charge. • Electron density is withdrawn inductively along the sigma bond, so the ring has less electron density than benzene, and thus it will be slower to react. Other Deactivators Halogens • Halogens are deactivators since they react slower than benzene. • Halogens are ortho, para-directors because the halogen can stabilize the sigma complex. Halogens Are Deactivators • Inductive effect: Halogens are deactivating because they are electronegative and can withdraw electron density from the ring along the sigma bond. Halogens Are Ortho, Para-Directors • Resonance effect: The lone pairs on the halogen can be used to stabilize the sigma complex by resonance. Energy Diagram Summary of Directing Effects Reduction of the Nitro Group • Treatment with zinc, tin, or iron in dilute acid will reduce the nitro to an amino group. • This is the best method for adding an amino group to the ring. Clemmensen Reduction • The Clemmensen reduction is a way to convert acylbenzenes to alkylbenzenes by treatment with aqueous HCl and amalgamated zinc. Wolff–Kishner Reduction • Forms hydrazone, then heat with strong base like KOH or potassium tert-butoxide • Use a high-boiling solvent: ethylene glycol, diethylene glycol, or DMSO. • A molecule of nitrogen is lost in the last steps of the reaction. Side-Chain Oxidation • Alkylbenzenes are oxidized to benzoic acid by heating in basic KMnO4 or heating in Na2Cr2O7/H2SO4. • The benzylic carbon will be oxidized to the carboxylic acid. Side-Chain Halogenation • The benzylic position is the most reactive. • Br2 reacts only at the benzylic position. • Cl2 is not as selective as bromination, so results in mixtures.