Chapter 5: Introduction to Reactions in Aqueous Solutions
advertisement

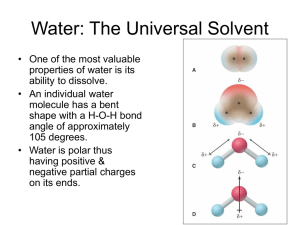
Chapter 5: Introduction to Reactions in Aqueous Solutions Electrolyte. A substance, such as sodium chloride, that dissolved in water and produces ions to give an electrically conducting solution is called an electrolyte. A substance, such as sucrose, or table sugar (C12H22O11), that is not ionized and does not conduct electric current when dissolved in water is called a Nonelectrolyte. Strong electrolyte: is a substance that is almost completely ionized in aqueous solution, and the solution is a good electrical conductor. Weak electrolyte: is partially ionized in aqueous solution, and the solution is only a fair conductor of electricity. (is an electrolyte that dissolves in water to give an equilibrium between a molecular substance and a relatively small quantity of ions.) NaCl(s) NH3(aq) + H2O(l) Na+(aq) + Cl-(aq) Strong electrolyte NH4+(aq) + OH-(aq) Weak electrolyte ~ Essentially all soluble ionic compounds and only a relatively few molecular compounds are strong electrolytes. ~ Most molecular compounds are either nonelectrolytes or weak electrolytes. Table 1 Electrolyte Classification of Some Common Substances Strong Electrolytes Weak Electrolytes Nonelectrolytes HCl, HBr, HI CH3COOH H2O HClO4 HF CH3OH HNO3 C2H5OH H2SO4 C12H22O11(sucrose) KBr Most organic compounds NaCl NaOH, KOH Other soluble ionic compounds E.g. Calculating ion concentrations in a solution of strong electrolyte. What are the concentration (in molarity) of Mg2+ and Cl- ions in 5.0g/L of MgCl2 solution? [Mg2+] = 5.0g x 1 molMgCl2 x 1 mol Mg2+ =5.3 x10-2 mol/L L 95.21gMgCl2 1 molMgCl2 [Cl-] =2 x 5.3x10-2 = 0.11 mol/L Aqueous Reactions Aqueous reactions can be grouped into three general categories, each with its own kind of driving force: precipitation reactions, acid base neutralization reactions, and oxidation-reduction reactions. Precipitation Reactions Precipitation reactions are process in which soluble reactants yield an insoluble solid product that falls out of solution. Most precipitations take place when certain cations and anions combined to produce an insoluble ionic solid called a precipitate. E.g. the reaction of silver nitrate and sodium iodide in an aqueous water solution yields sodium nitrate in solution and a yellow precipitate of silver iodide. We can write the equation for the reaction as follow: AgNO3(aq) + NaI(aq) AgI(s) + NaNO3(aq) we called this a molecular equation, an equation in which the substances are written as if they were molecular substances, even though they may exist as ions in the solution. Molecular equation is useful because it is explicit about what solutions have been added and what products are obtained; and to calculate the amount of reactants or products. Ionic Equations The molecular equation does not tell us that the reaction actual involves ions in solution. However, we know that soluble ionic substances in solution should be represented by their separate ions. To represent this, the above reaction as an ionic equation, in which all the ions are explicitly shown: An ionic equation: Ag+(aq) + NO3-(aq) + Na+(aq) + I-(aq) AgI(s) + Na+(aq)+NO3-(aq) This is an example of ionic equation, which is a chemical equation for a reaction involving ions in solution in which soluble substances are represented by the formulas of the predominant species in that solution. Net Ionic Equations Note that some ions appear on both side of equation. These ions go through the reaction unchanged- does not take part in the reaction. We called them spectator ions. We can cancel them from the equation. The resulting equation is a net ionic equation. Ag+(aq) + I-(aq) AgI(s) Net ionic Equation A net ionic equation is an equation that includes only the actual participants in a reaction, with each participant denoted by the symbol or formula that best represent it. rules for converting molecular equations to ionic equations • The rules for converting molecular equations to ionic equations follow: • 1) Make sure the molecular is balanced • 2) Ionic substances indicated in the molecular equation as dissolved in solution, such as NaCl(aq), are normally written as ions. • 3) Ionic substances that are insoluble (do not dissolve) either as reactants or products (such as precipitate) are represented by formulas of the compounds • 4) Molecular substances that are strong electrolytes, such as strong acids, are written as ions. Thus, HCl(aq) is written as H3O+(aq) + Cl-(aq) or as H+(aq) + Cl-(aq). • 5) Molecular substances that are weak electrolytes or nonelectrolytes are represented by their molecular formulas. Predicting Precipitation Reaction Empirical Rules for the solubilities of Common Ionic Compounds Soluble compounds Sodium, potassium and ammonium Exceptions compounds Acetate and nitrates Halides (chlorides, bromides, and iodides) Lead(II), silver and mercury(I) halides are insoluble Sulfates Calcium, strontium(Sr), barium and lead(II) sulfate are insoluble Insoluble compounds Carbonates and phosphates Exceptions Group 1 metals and ammonium compounds are soluble Hydroxides Group 1 metals compounds are soluble and calcium, Sr2+ and Ba2+ are slightly soluble Sulfides group 1 and group 2 metals and ammonium compounds are soluble _________________________________________________________________________________________________ E.g. Predict whether a reaction will occur in each of the following case. If so, write a net ionic equation for the reaction. If no reaction occurs, write NR after arrow. (a) Al2(SO4)3 + NaOH i) write down the reactants and interchange of anions to get product Al2(SO4)3 + 6NaOH2Al(OH)3 + 3Na2SO4 All common Na compounds are water soluble Na+ remain in solution. The combination of Al3+ and OH- produce insoluble Al(OH)3. Then the ionic equation is 2Al3+ +3SO42- + 6Na+ + 6OH-2Al(OH)3(s)+ 6Na+ + 3SO42The net ionic equation is : Al3+ + 3OH-Al(OH)3(s) (b) K2SO4(aq)+FeBr3(aq) (c) CdCl2(aq) + (NH4)2S(aq) Acid-Base Reactions Consider the production of ions in pure water. It produces a small percentage of ions (about 2x10-7% of the molecules react to gives ions). H2O(l)+ H2O(l) H3O+(aq) + OH-(aq) Arrhenius definition An acid is a substance that provides hydrogen ions (H+) (increase the concentration of H+) in aqueous solution. The symbol H+(aq) does not really represent the structure of the ion present in aqueous solution. H+ is too reactive to exit by itself, it attaches to water to give the more stable hydronium ion, H3O+. A base is a substance that produces hydroxide ions (OH-) (increase the conc. of hydroxide ions) in aqueous solution. HA (aq) H+(aq) + A-(aq) an acid HA is a general formula for an acid MOH(aq) M+(aq) + OH-(aq) an base MOH is a general formula for a base Strong Acid and Weak Acid A strong acid is an acid that is almost completely ionized in aqueous solution. A weak acid is an acid that only partially ionized (as result of an equilibrium reaction with water) in aqueous solution. E.g. HCl(aq) H+(aq) + Cl-(aq) strong acid HC2H3O2(aq) H+(aq) + C2H3O2-(aq) weak acid http://www.prenhall.com/petrucci Acids such as HCl and HNO3 that have only one acdic hydrogen atom per acid molecule are called monoprotic acids. A polyprotic acids such as H2SO4 and H3PO4 are acids that yield two or more acidic hydrogens per molecules. E.g. sulfuric acid, dissociate twice H2SO4(aq) + H2O(l) HSO4-(aq) + H3O+(aq) HSO4-(aq) + H2O(l) SO42-(aq) + H3O+(aq) Acid-Base Neutralization Reaction When a base is added to an acid solution, the acid is said to be neutralized. In a neutralization reaction, an acid and a base react to form water and an aqueous solution of an ionic compound called a salt. A neutralization reaction: HA(aq) + MOH(aq) H2O(l) + MA(aq) acid base water salt E.g. HCl(aq) + NaOH(aq) NaCl(aq) +H2O(l) H+(aq) + Cl-(aq) +Na+(aq) + OH-(aq) Na+(aq) +Cl-(aq) + H2O(l) By eliminating the spectator ions, we discover the natural of the neutralization of strong acid by a strong base The net ionic equation: H+(aq) + OH-(aq) H2O(l) or H3O+(aq) + OH-(aq) 2H2O(l) Strong Base and Weak Base A strong base is a base that dissociate nearly completely in aqueous solution. A weak base is a base that is only partially ionized (as result of an equilibrium reaction with water) in aqueous solution. E.g. NaOH(s) Na+(aq) + OH-(aq) strong base NH3(aq) + H2O(l) NH4+(aq) + OH-(aq) weak base As polyprotic acids can give more than one H+, some base yield more than one hydroxide ions. In a neutralization involving a strong acid and a weak base NH3 In a neutralization involving a weak acid and a strong base E.g NaOH(aq) + CH3COOH(aq) Reactions with Gases Formation Some reactions are driven to products by the formation of a gas. E.g carbonates react with acids to produce gases product. 1) NaHCO3(s) + HC2H3O2(aq) NaC2H3O2 (aq)+ H2CO3(aq) 2) Na2SO3(aq) + 2HCl(aq) 2NaCl + H2SO3(aq) 3) CaCO3(s) + 2H+(aq) Ca2+(aq) + H2CO3(aq) Oxidation-Reduction Reaction Historically, oxidation referred to the combination of an element with oxygen to yield an oxide, and the word reduction referred to the removal of oxygen from an oxide to yield the element. E.g. Rusting of iron: an oxidation of Fe 4Fe(s) + 3O2(g) 2Fe2O3(s) Manufacture of iron: a reduction of Fe 2Fe2O3 + 3C(s) 4Fe(s) + 3CO2(g) An oxidation and a reduction must occur together, and such a reaction is called an oxidation-reduction reaction or redox reaction. Today, by using broader definitions, we look at the change of oxidation state of the element involved in the reaction. An oxidation is defined as the lose of one or more electrons by a substance. A reduction is the gain of one or more electrons by a substance. A redox reaction is a process in which electrons are transferred between substance or in which atoms change oxidation number. Not all the redox reactions involve oxygen. How can you tell when a redox reaction is taking place? Oxidizing and Reducing Agents A species that is oxidized loses electrons or contain an atom that increases in O.S. #. Similarly, a species that is reduced gains electrons or contains an atom that decreases in oxidation #. An oxidizing agent: contains an element whose oxidation state decreases in a redox reaction (it make possible for some other substance to be oxidized and itself reduced.) gains electrons (electrons are found on the left side of its half-equation). A reducing agent: contains an element whose oxidation state increases in a redox reation loses electrons (electrons are found on the left side of its half-equation). In general, a substance with an element in one of its highest possible oxidation state is an oxidizing agent. If an element is in its lowest possible oxidation state, the substance is a reducing agent. Balancing Redox Reactions 1 The Oxidation-Number Method: the key to this method is to realize that the net change in the total of all oxidation numbers must be zero. That is, any increase in oxidation number for the oxidized atoms must be match by the corresponding decrease in oxidation number for the reduced atoms. E.g. Balance the following equation: MnO4¯(aq) + Br-(aq) Mn2+(aq) + Br2(aq) The next step is to find the net increase in oxidation number for oxidized atoms and the net decrease in oxidation number for the reduced atoms. Then, multiply the net increase and net decrease by suitable factors so that the two become equal. 2 Half Reactions Method A half reaction is one of two parts of an oxidation-reduction reaction, one of which involves a loss of electron and the other which involves a gain of electrons. Oxidation is a process in which the O.S. of some element increases (lose e-) and in which electrons appear on the right side of a halfequation. Reduction is a process in which the O.S. of some element decreases and in which electrons appear on the left side of a half-equation. Oxidation and reduction half-reactions must always occur together, and the total number of electrons associated with the oxidation must equal the total number associated with the reduction. 1) Write and balance separate half-equations for oxidation and reduction, and balance the equation. 2) Adjust coefficients in the two half-equation so that the same number of electrons appears in each half equation. 3) Add together the two half-equation, then cancel the species common to both side of the equation to obtain the balanced overall equation. Balancing the equation for redox reaction in acid solution. E.g. (a) Indicate whether each of the following is an oxidationreduction reaction. (b) balancing the equations. i) Fe2+(aq) + MnO4(aq) Fe3+(aq)+ Mn2+(aq) ii) H2PO4(aq) + OH-(aq) HPO42(aq) + H2O(l) Balance the equation basic solution E.g. MnO4 (aq)+ SO32 (aq) MnO2(s) + SO42 (aq) in basic solution 3 Disproportionation Reactions In some redox reactions, called disproportionation reactions, the same substance is both oxidized and reduced. E.g. Decomposition of hydrogen peroxide E.g. thiosulfate Stoichiometry of Reaction in Aqueous Solution: Titrations An important method for determining the amount of a particular substance is base on measuring volumes of reactant solution. Titration is a reaction carried out by the carefully controlled addition of one solution to another. The trick is stop at the point where both reactants have reacted completely, a condition called the equivalent point of the titration. E.g. A flask contains a solution of unknown amount of HCl. This solution is titrated with 0.101M NaOH. It take 3.35mL of NaOH to complete the reaction with HCl. What is the mass of HCl? E.g. A 0.235g sample of a solid that is 92.55 NaOH and 7.5% Ca(OH)2, by mass, requires 45.6mL of HCl(aq) solution for its titration. What is the molarity of the HCl(aq)?