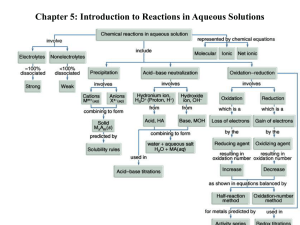
October 10th, 2012 - The Bronx High School of Science
advertisement

1) 2) Do Now: Compare Homework with a partner, how did you do? With your partner, write a list of steps on how to solve a limiting reagent problem to add to your notebook. • Define the word solution. – • How do you differentiate between the solute and the solvent in a solution? – • The solute is dissolved into the solvent Which is present in the smallest amount, the solute or the solvent? – • A homogenous mixture of two or more substances. Solute How do you determine if a solution is considered aqueous? – Solutions in which water is the solvent. A high percentage of naturally occurring chemical reactions occur with water as the solvent; why is water such an excellent solvent? Why is water considered a polar molecule? How does this benefit water a solvent? • How do we know, prior to testing for electricity, whether or not a substance will contain electrolytes? – • If a substance forms ions in solution. IE: NaCl. How does a nonelectrolyte differ from an electrolyte? – – May dissolve in water, but does not dissociate into ions. Electrolytes conduct electricity, non electrolytes do not. Ionic Compounds in Water • Summarize your observations of what occurred on the molecular level when solid NaCl was added to water. – – IONIC COMPOUND DISSOLVES IN WATER, IONS DISSOCIATE. EACH ION IS SURROUNDED BY SEVERAL WATER MOLECULES (AQUEOUS IONS “(aq)” » • • THESE IONS ARE CONSIDERED “SOLVATED” Illustrate this concept in your notebook. How is the electric current created? When a molecular compound (ie: CH3OH) dissolves in water, the solution usually consists of intact molecules dispersed homogeneously throughout the solution. There is nothing in solution to transport electric charge and therefor most molecular compounds are non-electrolytes **Important exceptions: ie: HCl, NH3 • • Compounds whose aqueous solutions conduct electricity well are called “Strong” Electrolytes. (exist in solution mostly as ions) Compounds whose aqueous solution conduct electricity poorly are called weak electrolytes NaCl(aq) Na+(aq) + Cl-(aq) • • Why is a single arrow used to show this dissociation? Soluble ionic compounds, strong acids, and soluble strong bases are considered strong electrolytes. Molecular compounds that produce small concentration of ions when dissolved = weak electrolytes. Ie: acetic acid, HC2H3O2, is primarily present in solution as molecules. Approx. 1 percent is present as ions. **Note: DO NOT confuse the extent to which an electrolyte dissolves with whether it is strong or weak.For example, HC2H3O2 is extremely soluble in water but is a weak electrolyte. In contrast, Ba(OH)2 is not very soluble, but the amount of the substance that does dissolve dissociates almost completely. Therefore, Ba(OH)2 is a strong electrolyte. How does this dissociation differ from the previous dissociation of NaCl? Why is the double arrow important in this equation? Do Now: Summarize yesterday’s lesson. • • How can we classify this type of reaction? Define the word precipitate. – Insoluble solid formed by a reaction in solution Ie: Pb(NO3)2 + 2KI → ?? – – Define the word solubility. • The amount of a particular substance that can be dissolved in a given quantity of solvent at that temperature. When does a substance become regarded as insoluble? • Substance with a solubility of less than 0.01 mol/L • N: NITRATES A: ACETATES G: GROUP 1 (ALKALI METALS) S: SULFATES (except PMS and CaStroBear) A: AMMONIUM G: GROUP 17 (except PMS) C: CARBONATES ( except for G1, Ammonium) A: ALCOHOLS (except for G1, CaStroBear, Ammonium) P: PHOSPHATES (except for G1, Ammonium) S: SULFIDE (except for G1, CaStroBear, Ammonium) • Are the following compounds soluble or insoluble and why? – – – – – – – KNO3 Li3PO4 AgCl NH4OH Ba3PO4 Hg2S Na2CO3 • Predict a general formula for an “exchange” or metathesis reaction. • • How would you describe an “exchange” reaction? • • • AX + BY → AY + BY Swapping of ions in solution Precipitation and acid-base reactions exhibit this pattern. Consider the following: 2KI(aq) + Pb(NO3)2(aq) → PbI2(s) 2KNO3(aq) – – • • Both Reactants are colorless solutions. When mixed, they form a bright yellow precipitate of PbI2 and a solution of KNO3. Final Product contains solid PbI2, Aqueous K+ ions and Aqueous NO3- ions. HOW MIGHT WE DIFFERENTIATE BETWEEN THE MOLECULAR, COMPLETE IONIC, and NET IONIC EQUATIONS? • Molecular: lists all species in complete chemical forms • • 2KI(aq) + Pb(NO3)2(aq) → PbI2(s) 2KNO3(aq) Complete Ionic: lists all strong electrolytes in rxn as ions • • • • Pb2+(aq) + 2NO3-(aq) + 2K+(aq) + 2I-(aq) → PbI2(s) + 2K+(aq) +2NO3(aq) Only strong electrolytes dissolved in solution are written in ionic form. Weak electrolytes and non-electrolytes are written in complete chemical form • Lists only ions which are not common on both sides of the equation Pb2+(aq) + 2NO3-(aq) + 2K+(aq) + 2I-(aq) → PbI2(s) + 2K+(aq) +2NO3-(aq) • Which ions can we remove from each side? – • 2NO3- and 2K+ Predict what the net ionic equation will look like? • Pb2+(aq) + 2I-(aq) → PbI2(s) • • • • How do we define spectator? Why are the ions we removed considered spectator ions? Formulate a step-by-step “how to” list for creating the net-ionic equation. 1) Write a balanced molecular equation 2) Rewrite equation to show ions that were present after dissociation (only strong electrolytes) 3) Identify and cancel “spectator” ions Complete the worksheet with a partner, be prepared to instruct the class on how you reached your answers. Define the word Acid. Substances that are able to ionize in aqueous solutions to form H+ Using this information, how might we define the word base? Proton acceptors How might we differentiate between a monoprotic acid and a diprotic acid? Monoprotic ionize to form 1 H+ ion. Diprotic ionizes to form 2 H+ ions. How soluble are strong acids and strong bases? Very. They completely ionize in solution. Strong Group 1A metal hydroxides, Ca(OH)2, Ba(OH)2 and Sr(OH)2 Strong Bases: Acids: HCl, HBr, HI, HClO3, H2SO4, and HNO3 Write the ionization of a strong acid. How does the ionization of a weak acid/base compare to that of strong acids/bases. Partially ionized in solution. HF(aq) is a weak acid; most acids are weak acids. Write the ionization of a weak acid. October 22nd, 2012 Do Now: How do you determine a weak acid/base. Write the Net Ionic Equation for the following reaction: When aqueous solutions of sodium phosphate and calcium chloride are mixed together, an insoluble white solid forms. Neutralization Reactions and Salts Write a generalized equation for a neutralization reaction. Acid + Base Water + Salt Define the word Salt. Any ionic compound whose cation (+) comes from a base and anion (-) comes from an acid. Example: Mg(OH)2(s) is a suspension and HCl is added. Write the net ionic equation. MgOH2(s) + 2H+ (aq) Mg2+ (aq) + 2H2O (l) Neutralization Reactions with Gas Formations How do sulfides act as bases? There are many bases besides OH- that react with H+ to form molecular compounds. The reaction of sulfides with acids give rise to H2S in gaseous form. Write the Net Ionic equation of Sodium Sulfide reacting Hydrochloric Acid. 2H+ (aq) + S2- (aq) H2S (g) Carbonates and hydrogen carbonates (or bicarbonates) will form CO2 (g) when treated with an acid. Write the net ionic equation of sodium bicarbonate reacting with Hydrochloric acid. H+ (aq) + HCO3- (aq) H2O (l) + CO2 (g) BOMBS AWAY! Oxidation-Reduction Reactions OXIDATION REDUCTION Oxidation-Reduction How is it determined which substance is undergoing oxidation vs. reduction? Loss of electrons = Oxidation Gain of electrons = Reduction Why are oxidation numbers useful? Oxidation numbers help us keep track of electrons during chemical reactions. How do we determine the oxidation numbers of atoms? RULES! I lost an electron! I’M POSITIVE! Hey! Why so Serious? What are you looking for? Are you sure?? RULES FOR OXIDATION STATES For an atom in its elemental state, the oxidation number is: For any monatomic ion, the oxidation number equals: The oxidation number of oxygen is usually: The major exception is in peroxides (containing the O22- ion) The oxidation state of Hydrogen is: The oxidation number of Flourine is: The sum of oxidation numbers in polyatomic a polyatomic ion: Practice determining oxidation state: Complete the worksheet handed out! Identifying Oxidation Vs. Reduction PRACTICE SHEET! Do Now: Find the oxidation states of each of the elements in the following compounds: 1. P2O5 2. NaH 3. C2O724. SnBr4 5. BaO2 How can we generalize a reaction between a metal or an acid and a salt? Write a general equation for this type of reaction. Define this type of reaction. Given this example: Zinc reacting with Hydrochloric acid, predict the products and determine which is being reduced and which is being oxidated. Write the molecular and net ionic equation for the following reaction: Zinc reacting with hydrobromic acid. Why is writing the net ionic equation helpful when discussing oxidation and reduction reactions? Complete 1 through 3 on a the worksheet given to you It is possible for a metal to be oxidized in the presence of a salt. Fe(s) + Ni(NO3)2(aq) Predict the products and write a molecular and net ionic equation for the reaction above. Will a metal always be oxidized in the presence of a salt? Explain your answer. The activity series lists metals in order of decreasing oxidation. How can we differentiate between active metals and noble metals? How do we determine if a reaction will occur using the activity series? Answer the aim in a summary paragraph. Do 1. 2. Now: It is said that HClO4 is a strong acid, whereas HClO2 is a weak acid. What does this mean in terms of the extent to which they ionize in solution? Classify each of the following as a nonelectrolyte, weak electrolyte, or strong electrolyte in water: 1. 2. 3. 4. H2SO3 C2H5OH (ethanol) NH3 KClO3 How can we differentiate between strong and concentrated solutions? Define How concentration do we express concentration of a solution? Molarity (symbol M) expresses concentration of solution (number of moles solute in a liter of solution) Calculate the molarity of a solution made by dissolving 23.4 grams of sodium sulfate in enough water to form 125 mL of solution. Calculate the molarity of a solution made by dissolving 5.00 grams of glucose in sufficient water to form exactly 100 mL of solution. Calculate the number of grams of solute in 0.250 L of 0.150 M KBr. When an ionic compound dissolves, the relative concentration of ions depend on the chemical formula of a compound. In a 1.0 M solution of NaCl, what are the concentration of Na+ ions and Cl- ions? In a 1.0 M solution of Na2SO4, what are the concentrations of the Na+ ions and the SO42ions? What is the molar concentration of K+ ions in a 0.015 M solution of potassium carbonate? Which will have the greatest concentration of potassium ion: 0.20 M KCl, 0.15 M K2CrO4, or 0.080 M K3PO4? If we know any two quantities involved in the molarity equation, we can solve for the third. Calculate the number of moles of HNO3 in 2.O L of a 0.200 M HNO3 solution. How do we dilute solutions? Moles solute in conc soln = moles dolute in dil solution We want to prepare 250.0 mL (convert to L) of 0.100 M CuSO4 solution by diluting a stock solution containing 1.00 M CuSO4. How many mL of concentration solution do we need? Do Now: How many milliliters of 3.0 M H2SO4 are needed to make 450 mL of 0.10 M H2SO4? What volume of 2.50 M lead (II) nitrate solution contains 0.0500 mol of Pb2+ ions? Two types of units exist: Laboratory Units: Chemical Units: How do you think these differ? Always convert laboratory units into chemical units first. Grams moles using molar mass Volume/molarity moles using M= mol/L Use Stoich. Coefficients to move between products and reactants. Convert lab units back into required units Why do we use titrations? How are titrations helpful in determining concentration? Suppose we know the molarity of an NaOH solution and we want to find the molarity of a given HCl solution. What do we already know? What do we want to know? How do we get there? How do we know when they are neutralized? Define Equivalence Point. After the titration is complete, how can we now solve for the molarity? What mass of NaCl is needed to precipitate the silver ions from a 20.0 mL of 0.100 M AgNO3 solution? How many mL of 0.120 M HCl are needed to completely neutralize 50.0 mL of 0.101 M Ba(OH)2 solution? If 42.7 mL of 0.208 M HCl solution is needed to neutralize a solution of Ca(OH)2, how many grams of Ca(OH)2 must be in the solution?