Chemical Reactions * Chapter 3
advertisement

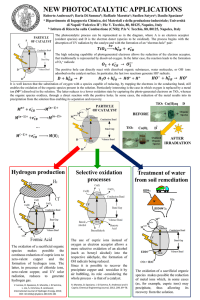
Chemical Reactions CHEMICAL REACTIONS EVIDENCES FOR CHEMICAL REACTIONS TYPES OF CHEMICAL REACTIONS Evidences for a Chemical Reaction COLOR CHANGE HEAT ABSORBED OR RELEASED FORMATION OF A PRECIPITATE CHEMICAL REACTION GAS RELEASE IRREVERSIBLE NEW PRODUCT FORMATION Evidence - A chemical change is accompanied by new product formation. Plants absorb water and Carbon-dioxide and make their own food in the presence of sunlight. This is known as “photosynthesis.” CO2 + H2O C6H1206 + O2 Evidence - A chemical change is irreversible. Evidence - A chemical change can be accompanied by gas release. We usually use, baking powder/baking soda in baking cakes and muffins. Baking powder/baking soda releases carbon-dioxide (gas) on heating and this makes our cakes and muffins rise. Evidence - A chemical change can be accompanied by color change. Leaves change color in Autumn from green to reddishorange and this is due to a chemical reaction. Evidence - A chemical change can be accompanied by heat release or absorption (exothermic vs endothermic). Burning of candles results in the release of heat to the surrounding (“exo”-outside and “thermic” – heat) and cold packs operate by means of a chemical reaction which absorbs heat from the surrounding (“endo” –inside and “thermic” –heat). Evidence - A chemical change can be accompanied by precipitate formation [precipitate = an insoluble solid compound formed when two soluble compounds are mixed with each other.] When vinegar is added to warm milk, it results in the removal of milk solids and this is known as “curdling of milk.” Curdling is a type of precipitation reaction. TYPES OF CHEMICAL REACTIONS SYNTHESIS REDOX DECOMPOSITION REPLACEMENT RXNS ACID-BASE COMBUSTION SINGLE DOUBLE Combustion reactions Image Courtesy of http://www.cdc.gov/eLCOSH/docs/d0400/d000485/imag e3.jpg Features of Combustion Reactions ● ● ● ● Fuel and oxygen required by all combustion reactions. If hydrocarbons (compounds containing carbon and hydrogen) are the fuel, then carbon-dioxide and water are formed as products. All combustion reactions are “exothermic” reactions as they release heat to the surroundings. Unless specifically stated, it is always assume combustion to be complete and predict products of complete combustion. Combustion Reactions contd • Combustion reactions always use oxygen of the air as a reactant. • Combustion of hydrocarbons (compounds containing hydrogen and carbon) produces CO2 and H2O. • H2O state whether gas or liquid depends on the temperature conditions of the reaction. • Products of incomplete combustion include CO and sometimes C also called soot. Synthesis Reactions • In synthesis, two or more substances react to form a single product. • Many elements react with one another to form compounds. • When a metal reacts with a non-metal, an ionic compound is formed. • 2 Mg + O2 • CaO + H2O 2 MgO Ca(OH)2 Decomposition • A single substance breaks apart to form two or more substances. • AB • Example: Sodium azide decomposes to form sodium and nitrogen. This reaction is used to inflate safety bags in automobiles. The system is designed in such a way that an impact results in ignition of sodium azide. • NaN3 A+B Na + N2 Single Replacement Reactions • A reaction in which one element replaces another element in a compound is called single replacement reaction. • In this type of reactions, a metal replaces a metal and a non-metal replaces a non-metal. • General equation for the reaction is: • A + BC AC + B • Activity Series- arrangement of elements in decreasing order of chemical reactivity. • Halogens – Each halogen will react to replace any of the halogens below it in the periodic table. • Cl2 + NaBr NaCl + Br2 • Metals – can be listed in a series in which each metal will replace all metals below it in the series and not he metals above. • Fe(s) + CuSO4(aq) • Fe(s) + MgSO4(aq) FeSO4 + Cu N.R (No Reaction) Precipitation Reactions • Also called metathesis or exchange reactions. • Two soluble ionic compounds react to form an insoluble product called a precipitate. • Why does a precipitate form? • The electrostatic force of attraction outweighs the tendency of the ions to remain solvated so the ions cluster together and come out of the solution as a solid. • Solvation is a process where ions freely move about in solution. This is possible because most ionic compounds are soluble in nature and dissociate in solution to form the corresponding ions. • How to predict whether precipitation occurs or not? • Note the ions present. • Consider the possible cation-anion combinations. • Decide whether any combination is insoluble by referring to the solubility chart. • Write both ionic and molecular equations. • Aqueous solutions of silver nitrate and barium chloride mixed together. • The ions present are Ag+ (silver), Ba2+ (barium), Cl-1 (chloride) and nitrate (NO3-1). • The possible combinations include AgCl (silver chloride), Ba(NO3)2. • All nitrates are soluble but silver chloride is insoluble. • Molecular Equation: • AgNO3 + BaCl2 AgCl + Ba(NO3)2 Acid-Base Reactions • Arrhenius definition – Acid is a substance which releases H+1 ions in solution. • A base is a substance which releases OH-1 ions in solution. • When an acid and base react, the H+1 and OH-1 ions combine to form neutral H2O molecules. • The reaction between an acid and a base is called a neutralization reaction. • Acids and bases, in general, are referred to as “electrolytes.” • Strong acids and bases completely dissociate into ions whereas weak acids/bases partially dissociate into ions. • A solution of acid, base can conduct electricity. • Common acids/bases in household • Tartaric acid – grapes • Citric acid – citrus fruits • Vinegar or acetic acid • Sting of bees and insects – formic acid • Milk of magnesia – Magnesium hydroxide • Mylanta – Magnesium hydroxide and Aluminum hydroxide • Viewing acid-base reactions as proton-transfer reactions. • An acid is a proton donor and a base is a proton acceptor. (Lowry-Bronsted theory) • Example – gas forming reactions. • Reaction between an ionic carbonate and an acid • Such reactions result in the formation of a gas (carbon-dioxide) and water. Acid – Base Titrations • Titration – quantitative procedure. • One solution of known concentration is used to determine the concentration of another solution through a monitored reaction. • Solution whose concentration is known is called a standardized solution. • A standardized solution is one whose concentration is known. • Usually the base is chosen as the standardized solution. • The acid concentration has to be determined. • A few drops of indicator added to the acid solution. • An indicator is a chemical substance which changes color in the presence of an acid or a base. • Examples of indicator – phenolphthalein, bromothymol blue. • The standardized solution is slowly added from the buret to the acid solution of unknown concentration. • The solution changes color when all the acid is consumed by the base and a few drops of excess is present. • Equivalence point – in a titration is when all moles of H+1 present in the solution have reacted with all moles of OH-1 added from the buret. • End point – in a titration is when a tiny excess of base is added to change the color of the indicator. • Usually end point and equivalence point are close to each other. Redox Reactions Oxidation – a process which involves loss of electrons. Reduction – a process which involves gain of electrons. Oxidizing agent – a substance which helps oxidation take place – removes electrons from a different substance and as a result gets reduced. Reducing agent – a substance which helps reduction take place – donates electrons to a different substance and as a result is oxidized. • Use oxidation number to monitor the movement of electrons. • Oxidation number /oxidation state tells us the number of electrons gained or lost by an atom. • Rules for assigning oxidation number• All elements in group 1 have a +1 oxidation # in all compounds. • All elements in group 2 have a +2 oxidation # in all compounds. • Hydrogen has a +1 oxidation # in combination with all nonmetals and a -1 oxidation # in combination with metals and boron. • Fluorine has a -1 combination in all compounds. • All other halogens have a -1 in combination with metals, nonmetals (except O) and other halogens lower in the group.) • Oxygen has an oxidation # of -2 in all compounds except in combination with fluorine.