ch 4 reactions in solutions power point
advertisement

UU Chemistry 216 chapter 4
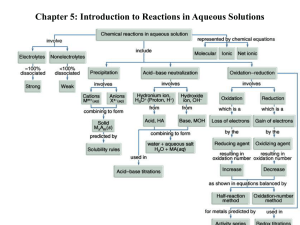
Reactions in aqueous solutions
Introduction: Reactions in aqueous
solutions
• A large amount of important chemistry takes place in water
– aqueous solutions
• Precipitation reaction- Soluble reactants yield an insoluble
product:
Pb(NO3)2(aq) + 2KI (aq) -> 2KNO3(aq) + PbI2(s)
• Acid – Base neutralization reactions – acid reacts with base
to yield water and a salt.
HCl(aq) + NaOH(aq) H2O (l) + NaCl(aq)
• Oxidation – reduction reactions – electrons are transferred
between reaction partners. Shown by change of charges /
oxidation numbers
Mg(s) +2HCl(aq) MgCl2 (aq)+ H2(g)
Water solutions
• Molecular substances (sugar) in water it contains
neutral sucrose molecules surrounded by water.
Nonelectrolyte.
• Ionic substances (salt) when dissolves in water,
the solutions contain separate Na+ and Cl- ions
(dissociates) surrounded by water. Electrolyte. If
dissociates a large extent (70 – 100%) then strong
electrolyte - KCl. Small extent then weak
electrolyte – acetic acid (CH3CO2H). Use a double
arrow to indicate reaction goes both directions.
Goes until equilibrium is reached.
(molecules and ion formation)
Electrolyte classification
• Strong electrolytes: HCl, HBr, HI, HClO4, HNO3,
N2SO4, KBr, NaCl, NaOH, KOH
• Weak: acetic acid, HF
• Nonelectrolytes: H2O, methyl alcohol, ethyl
alcohol, sucrose, most compound of carbon
(organics)
• Beaker with solution – wires / battery / bulb
Precipitation reactions
• We’ve been writing molecular equationssubstances written using their complete formulas
as if they were molecules. For ionic’s it is more
accurate to write precipitation reactions as ionic
equations.
Pb+2 + 2NO3- + 2K+ +2I- -> 2 K+ + 2 NO3- +PbI2 (s)
Spectator ions (NO3- and K+) undergo no change,
balance the charge, found on both sides of
equation.
Net ionic shows only the ions undergoing change
Calculations: [concentration] ions in
solution
• What is the total molar concentration of ions
in a 0.350 M solutions of the strong
electrolyte Na2SO4?
Properties of water
• Only substance that exists in large quantities in all
three states (solid, liquid, gas)
• Less dense as a solid than a liquid
• Polar molecule due to electronegativity of oxygen
• Hydrogen bonding – Hydrogen of one compound
bond with O, F, N of another
• Surface tension – force needed to overcome
intermolecular attraction, cohesion
• Capillary action- adhesion
• High specific heat – raise temp. of 1 g, 1*C
Calculation: Writing a net ionic
equation
• Aqueous hydrochloric acid reacts with zinc
metal to yield hydrogen gas and aqueous zinc
chloride. Write a net ionic equation for the
process.
Solubility
• To predict whether a precipitation reaction will
occur you must know the probable solubility of
each potential product.
• 1. A compound is probably soluble if it contains
one of the following cations: Group 1; ammonium
• 2. A compound is probably soluble if it contains
one of the following anions: Halides – Cl, Br, I
except Ag, Hg2,Pb; Nitrate; perchlorate; acetate,
sulfate – except Ba,Hg2, Pb, Ca
• 3. If not contains one of above, then usually not
soluble
Calculation: predict products
• Will a precipitation reaction occur when
aqueous solutions of CdCl2 and (NH4)2S are
mixed? If so, write the net ionic equation.
Calculations: Using a precipitation
reaction to prepare a substance
• How might you use a precipitation reaction to
prepare a sample of CuCO3? Write net ionic
equation.
Calculation: Identify precipitation
reactions
• Predict if the compound is likely to be soluble.
a. CdCO3
b. Na2S
• Predict whether a precipitation reaction will
occur. Write a net ionic equation for it.
NiCl2 (aq)+ (NH4)2S(aq)
Acid, base history
• Antoine Lavoisier- acids contain a common
element: oxygen
• Sir Humphrey Davy – muriatic acid (HCl), not
oxygen but Hydrogen
• Svante Arrhenius – acids dissociate in water to
produce hydrogen ions, bases hydroxide ions.
• Bronsted – Lowery – acids donate a proton
(Hydrogen ion – from Hydronium ion), base is
proton acceptor
Acids – Bases neutralization:
acid properties
• Produce hydrogen ions in water, a sour taste,
Corrode some metals, Turn blue litmus red,
Are electrolytes, Neutralizes bases
• Strong acids – dissociates completely into ions
• Perchloric acid, sulfuric acid, Hydrobromic
acid, Hydrochloric acid, Nitric acid
• Weak acid – do not ionize very much
• Phosphoric acid, Hydrofluoric acid, acetic acid
Acid – Base Neutralization:
Base properties
• Produce hydroxide ions in water, Taste bitter,
Feel slippery, soapy, Turn red litmus blue, Are
electrolytes, Neutralizes acids
• Strong bases - separates completely into
metal ions and hydroxide ions.
• Sodium hydroxide, Potassium hydroxide,
Barium hydroxide, Calcium hydroxide
• Weak base – only a few hydroxide ions
• Ammonia
Kw and the pH scale
• Kw (ion product of water) is a constant formed
from the multiplication of the hydronium and
hydroxide concentration in pure water
• Kw = [H3O+] [OH-]
• (1 X 10 -14M) = (1 x 10-7M) (1 x 10-7M)
• pH scale = - log [H3O+] : the log of a base 10 is the
exponent. The negative turn it to a positive.
• Strong acid 0 – 6 weak acid {7 neutral} weak base
8 – 14 strong base
Calculation pH
[H30+]
[OH-]
pH
1 x 10 -8
1 x 10 -9
7
Acid / base
/neutral
Acid – Base titration
• Titration is a procedure for determining the
concentration of a solution by allowing a carefully
measured volume to react with a solution of
another substance (standard solution) whose
concentration is known.
• Measure out a known volume of unknown
concentration - HCl. Add indicator . Fill buret
with known concentration of NaOH. Add until
indicator (phenolphthalein) just begins to turn
pink. Read volume of NaOH and calculate
molarity of HCl.
Calculations: acid – base titrations
• NaOH + HCl NaCl + H2O
• If we take 20.0 mL of a HCl solution and find
that we have to add 48.6 mL of a 0.1 M NaOH
from a buret to obtain complete reaction,
what is the molarity of the HCl?
Acid – Base neutralization
• When an acid and a base are mixed in the
right stoichiometric proportions, both acid
and basic properties disappear because of a
neutralization reaction, producing water and a
salt. The anion of salt (A-) comes from the
acid, the cation of salt (M+) comes from the
base.
• HA (aq) + MOH (aq) H2O (l) + MA (aq)
Acid plus base yields water and salt
Calculations: writing ionic and net
ionic equations
• Write both an ionic equation and a net ionic
equation for the neutralization reaction of
aqueous HBr and aqueous Ba(OH)2.
Buffers
• Resists a change in pH when small amounts of
acid or base are added.
• Must contain a weak acid and a salt of that acid
OR weak base and a salt of that base.
• Example: For blood (7.4 pH) – weak acid
(carbonic acid) and salt of that acid (sodium
bicarbonate). Add hydroxide (base) to blood and
weak acid neutralizes it. Add hydronium ion
(acid) to blood, it reacts with bicarbonate ion in
blood to form carbonic acid.
Oxidation – Reduction (Redox):
oxidation numbers
•
•
•
•
Oxidation is the loss of one or more electrons.
Reduction is the gain of one or more electrons
Oxidize A-2 – A-1 – A – A+ – A+2 Reduce
The superscripts are now called oxidations
number. It’s not always the same value as the
ionic charge.
• Rules for assigning oxidation numbers:
Oxidation rules
• 1. An atom in its elemental state has an
oxidation number of 0. (Na, H2, Br2, S, Ne)
• 2. An atom in a monatomic ion has an
oxidation number identical to its charge.
(Na+1, Ca+2, Al+3, O -2, Cl-1)
• 3. An atom in a polyatomic ion or in a
molecular compound usually has the same
oxidation number it would have if it were a
monatomic ion. (H – O – H, [O-H]-1)
Oxidation rules con’t.
• A. Hydrogen can be either +1 or -1 (when
bonded to a metal like Calcium)
• B. Oxygen usually has an oxidation number of
-2 (peroxides [O2-2, O-O; oxygen is -1 )
• C. Halogens usually have an oxidation number
of -1. (bonded to oxygen, +1 : Cl – O - Cl)
• 4. The sum of the oxidation numbers is 0 for a
neutral compound and is equal to the net
charge for a polyatomic number.
Calculations: Assigning oxidation
numbers
• Assign oxidation numbers to each atom in the
following substances.
• A) CdS –
• B) AlH3• C) S2O3-2• D) Na2Cr2O7-
Identify redox reactions
• 4 Fe(s) + 3 O2 (g) 2 Fe2O3 (s) (g)
• Assign oxidations numbers, and if a change
(more positive / less negative – oxidation; less
positive / more negative – reduction) Both
always occur together.
• Fe goes from 0 to +3 – loses 3 electron (x 4 by
coefficient = 12 electrons total)
• Oxygen from O to -2 (x2 for diatomic, x 3 by
coefficient = 12 electrons total)
Oxidizing and reducing agents
• Reducing agents – Cause reduction, loses one
or more electrons, undergoes oxidation,
oxidation number of atoms increases. In
general metals.
• Oxidizing agent – causes oxidation, gains one
or more electrons, undergoes reduction,
oxidation number of atom decreases. In
general reactive nonmetals such as oxygen
and the halogens.
Calculations: identify oxidizing and
reducing agents
• Assign oxidation numbers to all atoms, tell in
each case which substance is undergoing
oxidation and which reduction, and identify
the oxidizing and reducing agents.
• Ca(s) + 2 H+ (aq) Ca+2 (aq) + H2 (g)
• 2 Fe+2 (aq) + Cl2 (aq) 2 Fe+3 (aq) + 2 Cl- (aq)
Activity series
• Whether or not a reaction occurs between a
given ion and a given element depends on the
relative ease with which they gain or lose
electron. It’s possible to construct an activity
series which rank the elements in order of
their reducing ability in aqueous solution.
Those on top give up electrons readily and are
reducing agents, those at bottom give up
electrons less. Any element higher in the
activity series will reduce the ion of element
below
A partial activity series of the
elements
• Strongly reducing: Li, K, Ba, Ca, Na, (these
element react rapidly with acids or liquid
water to release hydrogen gas)
• Mg, Al, Mn, Zn, Cr, Fe, (these react with acids
or steam to release hydrogen gas)
• Co, Ni, Sn, (These react with acids to release
hydrogen gas)
• H2
• Rest does not react with acids Cu, Ag, Hg, Pt,
Au : Weakly reducing
Calculations: predicting the product of
redox reactions
• Predict whether the following redox reactions
will occur.
• A) Hg+2 (aq) + Zn (s) Hg (l) + Zn+2 (aq)
• B) 2 H+ (aq) + 2 Ag (s) H2 (g) + 2 Ag+ (aq)
Balancing Redox: oxidation – number
method
• 1. Write the unbalanced net ionic equation.
• 2. Balance the equation for all atoms other
than H and O.
• 3. Assign oxidation numbers to all atoms.
• 4. Decide which atoms have changed
oxidation numbers and by how much
• 5. Make the total increase in oxidation
number for oxidizing atoms equal to the total
decrease in oxidation number for reduced.
Balancing Redox: oxidation –
number method con’t
• 6. Balance the equation for O by adding water
to the side with less O and then balance for H
by adding H+ to the side with less H.
• 7. (for bases) Add OH- (to balance H+) to each
side of the product. This will “neutralize“ the
H+, giving water on product and base in
reactant side.
• 8. (for bases) Cancel out extra waters that
occurs on both sides. This is your final net
ionic equation that is balanced for charges.
Calculation: Balancing a reaction in a
acid and bases
• Potassium permanganate (KMnO4) with
sodium bromide in aqueous solution.
• MnO4- (aq) + Br- (aq) Mn+2 (aq) + Br2 (aq)
• Potassium permanganate reacts with aqueous
sodium sulfite (Na2SO3) in basic solution to
yield the green manganate and sulfate ion.
• MnO4- (aq) + SO3-2 (aq) MnO4-2 (aq) + SO4-2
(aq)
Balancing Redox Reactions: the halfreaction method.
• 1. Write the unbalanced net ionic equation
• 2. Decide which atoms are oxidized and which
are reduced, and write the two unbalanced
half-reaction.
• 3. Balance both half-reactions for all atoms
except O and H.
• 4. Balance each half-reactions for O by adding
water to the side with less O, and balance for
H by adding H+ to the side with less H.
Balancing Redox Reactions: the
half-reaction method
• 5. Balance each half-reaction for charge by
adding electrons to the side with greater
positive charge, and then multiply be suitable
factors to make the electrons count the same in
both half – reactions
• 6. Add the two balanced half-reactions together,
and cancel electrons and other species that
appear on both sides of the equation.
• If Base, add OH- to both side to neutralize H+
Calculation: writing half - reactions
• A) Mn+2(aq) + ClO3-(aq) MnO2(s) + ClO2(aq)
• B) Cr2O7-2(q) + Fe+2(aq) Cr+3(aq) + Fe+3(aq)
Calculation: Balancing an equation
in an acid
• Aqueous potassium dichromate (K2Cr2O7) with
aqueous NaCl.
• Cr2O7-2 (aq) + Cl- (aq) Cr+3 (aq) + Cl2 (aq)
Calculations: Balancing a reaction in a
base
• Aqueous sodium hypochlorite (NaOCl) is a
strong oxidizer that reacts with chromite ion
[Cr(OH)4-] in a basic solution to yield chromate
(CrO4-2 ) and chloride ion.
• ClO- (aq) + Cr(OH)4- (aq) CrO4-2 (aq) + Cl- (aq)
Redox titration
• Very similar to acid / base titration. The
substance whose concentration you want to
determine undergo an oxidation or reduction
reaction is 100% and there be some means
(color change) to indicate the reaction is
complete. Indicator maybe in the reaction
itself or a due to added redox indicator.
Calculations: Using redox titration to
determine a solution’s concentration
• The concentration of an aqueous I3- solution
can be determined by titration with aqueous
sodium thiosulfate (Na2S2O3) in the presence
of a starch indicator (turns from blue to
colorless when all I3- has reacted). What is the
molar concentration of I3- if 24.55mL of a
0.102M Na2S2O3 is needed for complete
reaction with 10.0 mL of I3- solution.
• 2S2O3-2 (aq) + I3- (aq) S4O6-2 (aq) + 3I- (aq)
Redox applications
• Combustion – burning of carbon hydrogen
(fuel) with oxygen in air.
• Bleaching – redox to decolorize / lighten
materials. Hydrogen peroxide (H2O2) for hair,
sodium hypochlorite (NaOCl) for clothes,
elemental chlorine for wood pulp
• Metallurgy – extracting and purifying metals
form ores. Zinc is made from reduction of ZnO
with coke (carbon).
• ZnO(s) + C(s) Zn(s) + CO(g)
More Redox application
• Batteries – Zinc, ammonium chloride paste,
MnO2 paste (graphite rod sticks) are connected
by wires, sending electrons flowing through the
wire toward the MnO2.
• Zn(s) + 2 MnO2(s) + 2NH4Cl(s) ZnCl2(aq) +
Mn2O3(s) + 2NH3(aq) + H2O(l)
• Corrosion – is the deterioration of a metal by
oxidation (rust). ¼ Fe used to replace rusted.
• 4 Fe(s) + 3O2(g) –(H2O) 2Fe2O3*H2O(s)