Water: The Universal Solvent
advertisement

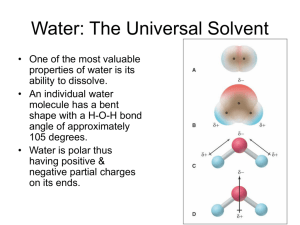
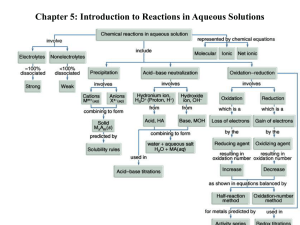
Water: The Universal Solvent • One of the most valuable properties of water is its ability to dissolve. • An individual water molecule has a bent shape with a H-O-H bond angle of approximately 105 degrees. • Water is polar thus having positive & negative partial charges on its ends. Ionic Compounds in Water • The positive ends of a water molecule are attracted to negative cations and the negative ends are attracted to positive cations in an ionic compound – this is called hydration. • The ions become hydrated & move around independently. Covalent Compounds in Water • Water also dissolves many nonionic substances such as ethanol (C2H5OH). • The reason for this is that ethanol is also polar. • Polar dissolves polar – “like dissolves like”. • This is the reason water will not dissolve oil. Solutions as Electrolytes • Strong conductor = strong electrolyte • Weak conductor = weak electrolyte • No conductor - nonelectrolyte Solution Concentration • Solution can be expressed in a variety of ways but the most common method is molarity: M = moles solute volume (L) solution • Example: calculate the molarity of a solution prepped by dissolving 1.56 g of gaseous hydrochloric acid in enough water to make 26.8 mL of solution. – 1.60 M HCl Concentration of Ions in Solution • • (a) (a) When an ionic salt dissolves in water ions are form in solution. The moles of ions formed must be considered in concentration. Example: what is the concentration each ion in (a) 0.50M cobalt II nitrate (b) 2.0M iron III perchlorate. Co(NO3)2 (s) + H2O Co2+ (aq) + 2NO3- (aq) [Co2+] = 1 x 0.50M = 0.50M [NO3-] = 2 x 0.50M = 1.0M Fe(ClO4)3 (s) + H2O Fe3+ (aq) + 3ClO4- (aq) [Fe3+] = 2.0M [ClO4-] = 6.0M Concentration of Ions in Solution • Example: Calculate the number of moles of chloride ions in 1.75 L of 1.0 x 10-3M zinc chloride. (step 1) ZnCl2 (s) Zn2+(aq) + 2Cl-(aq) (step 2) [Cl-] = 2 x 1.0 x 10-3 = 2.0 x 10-3M (step 3) 1.75 L x 2.0 x 10-3 mole Cl- = 1L 3.5 x 10-3 moles of chloride ions Example: Concentration & Volume • Typical blood serum is about 0.14M sodium chloride. What volume of blood contains 1.0 mg of sodium chloride? – 0.12 mL of blood • To analyze the alcohol content of a certain wine, a chemist needs 50.00 mL of an aqueous 0.200M potassium dichromate solution. How much solid potassium dichromate must be weighed out to make this solution? – 2.94 g K2Cr2O7 Dilution Formula • This formula allows a chemist to prepare a diluted solution from a concentrated one. M1V1 = M2V2 or McVc = MdVd • Example: what volume of 16 M sulfuric acid must be used to prepare 1.5 L of a 0.10 M sulfuric acid solution? – 9.4 mL of H2SO4 must be diluted with 1.5 L of water. Types of Chemical Reactions Precipitation Reactions • When 2 aqueous solutions are mixed an insoluble precipitate sometimes forms – also known as double replacement or metathesis reactions. • It is important to remember that some ions are the key players and some are just spectators: – The formula equation gives the overall reaction. – The complete ionic equation represents all ions involved in the reaction. – The net ionic equation includes only those solution components undergoing a change, spectator ions are not included. The reaction of Pb(NO3)2 & NaI. Write the formula equation, ionic & net ionic equations: Stoichiometry of Precipitation Reactions • Calculate the mass of solid sodium chloride that must be added to 1.50 L of a 0.100 M silver nitrate solution to precipitate all the silver ions in the form of silver chloride. • First find the # of moles Na necessary for the # of moles of silver ions already present, then convert to grams. – 8.77 g NaCl Example: Determine Mass of Product Formed • When aqueous solutions of sodium sulfate and lead II nitrate are mixed a precipitate forms. Give the net ionic equation for the reactions and calculate the mass of this precipitate when 1.25 L of 0.0500 M lead II nitrate and 2.00 L of 0.0250 M sodium sulfate. 15.2 g PbSO4 Acid-Base Reactions • An acid is a substance that produces H+ ions when dissolved in water. • A base is a substance that produces OH− ions when dissolved in water. Acids and bases as Electrolytes A, Strong acids and bases are strong electrolytes, as indicated by the brightly lit bulb. B, Weak acids and bases are weak electrolytes. Neutralization Reactions Strong Acid—Strong Base: Because both ionize completely, the H+ ions and OH- ions react with each other to form water molecules. Basic: HNO3 (aq) + NaOH (aq) Ionic: H+ (aq) + NO3- (aq) + Na+ (aq) + OH- (aq) Net Ionic: H+ (aq) + OH- (aq) H2O (l) Weak Acid – Strong Base: A two-step process occurs: • 1. The ionization of the weak acid HB (aq) H+ (aq) + B- (aq) • 2. The neutralization of the H+ ion by the OH- from the strong base H+ (aq) + OH- (aq) H2O (l) Net Ionic: HX + OH- X- + H2O Strong Acid – Weak Base: This is also a two-step process: • 1.The first step occurs when the weak base reacts with water to produce OHions. NH3 + H2O NH4+ + OH- • 2. In the second step the H+ ions from the strong acid neutralize the OH- ions to form water. H+ + OH- H2O • Net Ionic: H+ + X XH+ Example: Neutralization Reactions • 1)What volume of a 0.100 M HCl is needed to neutralize 25.0 mL of 0.350 M NaOH? 8.75 x 10-2 L • 2)In a certain experiment, 28.0 mL of 0.250 M nitric acid and 53.0 mL of 0.320 M potassium hydroxide are mixed. Calculate the moles of water formed in the resulting reaction. What is the [H+] and [OH-] after the reaction goes to completion? 0.024 moles H2O, [H+] = 0, [OH-] = 0.123 M Acid-Base Titration • This is a volumetric analysis technique for determining the amount (usually concentration) of a substance. • This involves the delivery (from a buret) of a measured volume of a solution of known concentration (the titrant) into a solution containing the substance being analyzed (the analyte). • The neutralization point is known as the equivalence point. This point is marked by an indicator. • The point when the indicator changes color is known as the end point. • The goal is to choose an indicator which has a similar endpoint as the equivalence point your reaction. Titration Example #1 You perform an acid-base titration to standardize an HCl solution by placing 50.00 mL of HCl in a flask with a few drops of indicator solution. You put 0.1524 M NaOH into the buret, and the initial reading is 0.55 mL. At the end point, the buret reading is 33.87 mL. What is the concentration of the HCl solution? Titration Example #2 In a titration, it is found that 25.0 mL of 0.500 M NaOH is required to react with • (a) a 15.0-mL sample of HCl. What is the molarity of HCl? 0.833 M • (b) a 15.0-mL sample of a weak acid, H2A. What is the molarity of H2A, assuming the reaction to be H2A(aq) + 2OH-(aq) 2H2O + A2-(aq)? 0.417 M • (c) an aspirin tablet weighing 2.50 g. What is the percentage of acetylsalicylic acid, HC9H7O4, in the aspirin tablet? The reaction is HC9H7O4 (s) + OH- (aq) H2O + C9H7O4 - (aq) 90.0% Oxidation-Reduction (Redox) • Oxidation means the losing of electrons (an increase in the oxidation #) and reduction means the gaining of electrons (a decrease in the oxidation #). The 2 occur together, they are opposite sides of the same coin. • A good way to remember LEO the lion goes GER – losing electrons oxidation…..gaining electrons reduction OR OIL RIG – oxidation is losing, reduction is gaining. Oxidizing & Reducing Agents • An oxidizing agent is the species that accepts the electrons i.e. the H+ ion above. – Non-metals tend to be oxidizing agents. • A reducing agent is the species that donates the electrons i.e. the Zn above. – Metals tend to be reducing agents. Oxidation Numbers • The first step to balancing any redox is assigning oxidation numbers to reactants and products in the equation – please reference pg. 89 in text: – The oxidation # of an element in its elemental state is 0. – The oxidation # of an element in a monatomic ion is equal to the charge of that ion. – Certain elements have the same oxidation in all or almost all their compounds. – The sum of the oxidation numbers in a neutral species is 0; in a polyatomic ion, it is equal to the charge of that ion. Assigning Oxidation #’s Practice • What is the oxidation number of phosphorus – in sodium phosphate? • P = +5 – In the dihydrogen phosphate ion? • P = +5 Balancing Redox Reactions • • Assign oxidation numbers (Rules – Pg. 89) Identify the oxidation and reduction reactions. Split in 2 half reactions. Balance the element being oxidized and reduced. Balance the elements (that is being reduced or oxidized) oxidation # by adding electrons. Oxidation adds to the right, reduction adds to the left. Balance the oxygens by adding water molecules. Balance the hydrogens by adding H+ ions. If the electrons on both sides are not the same you must find the least common multiple between the 2 electrons. Multiply each reaction to get the same number of electrons on both sides. • • • • • – • • It is important to check that all atoms and charges balance at this point. THE MISTAKES ARE LIKELY TO HAPPEN HERE!!! Combine the reactions and simplify if necessary. If in basic solution add OH- ions to both sides to produce water molecules on the side with H+ ions. Simplify the water molecules if necessary. Zn (s) + 2HCl (aq) ZnCl2 (aq) + H2 (g) • Oxidation: • Reduction: Zn Zn+2 + 2e2H+ + 2e- H2 Net ionic equation: Zn (s) + 2H+ (aq) H2 (g) + Zn+2 (aq) Balance the following redox… • (a) Fe2+(aq)+ MnO4- (aq) Fe3+(aq) Mn2+(aq) (acidic solution) • (b) Cl2(g) Cr(OH)3(s) Cl- (aq) CrO42- (aq) (basic solution)