RNA Synthesis and Splicing
advertisement

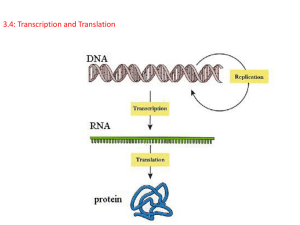
Transcription and Splicing machinery DNA primary mRNA mature mRNA Transcription + Processing 1 Prokaryotic and Eukaryotic RNA Polymerases are similar in shape Sigma (σ) subunit missing -> Different number of subunits 2 Recognizes the promoter site (-10 box + -35 box) 3 RNA polymerase mechanism -> Similar to DNA polymerase -> 3’-hydroxyl group of RNA chain attacks the a-phosphoryl group of the incoming NTP -> Transition state stabilized by Mg2+ 4 Transcription AFM image of short DNA fragment with RNA polymerase molecule bound to transcription recognition site. 238nm scan size. Courtesy of Bustamante Lab, Chemistry Department, University of Oregon, Eugene OR 5 6 7 Prokaryotic promoter sites -35 -10 +1 5’-----TTGACA--------------TATAAT---------start site----3’ σ subunit 8 Prokaryotic promoter sites σ subunit interacts with -10 box and -35 box Alternative E. coli promoters Stanard Promoter -> σ70 Heat shock promoter -> σ32 N-starvation promoter -> σ54 10 Footprinting 11 DNA unwinding prior to Initiation of Transcription -> Transition from closed to open complex -> Unwinding done by RNA polymerase 1 RNA polymerase molecule -> 17bp segment -> 1.6 turns on B-DNA 12 Negative supercoiled DNA favors the transcription -> neg. supercoiling facilitates unwinding -> introduction of neg. supercoiling -> increases rate of transcription -> Exception -> promoter of TopoII -> neg. Supercoiling -> decreases rate of transcription 13 Transcription bubble First Nucleotide is pppG or pppA -> Transcription start 14 RNA-DNA hybrid separation RNA polymerase forces the separation of the RNA-DNA hybrid 15 Transcription Termination Rho independent termination -> RNA polymerase pauses after production of hairpin -> RNA-DNA hybrid of hairpin is unstable => RNA falls off Termination by Rho protein Rho interacts with RNA polymerase -> breaks the RNA-DNA hybrid helix -> functions as a helicase 16 Primary transcript of rRNA is modified Modification: 1. Cleavage of primary transcript by Ribonuclease III 2. Modification of bases (Prokaryotes: methylation) and ribose (Eukaryotes: methylation) 17 tRNA transcript is also modified Modification: 1. Cleavage of primary transcript by Ribonuclease III 2. Addition of nucleotides at 3’ end (CCA) 3. Unusual bases 18 tRNA transcript processing Modification: 1. Cleavage of primary transcript by Ribonuclease III 2. Addition of nucleotides at 3’ end (CCA) 3. Unusual bases 19 Antibiotic Inhibitors of Transcription Rifampicin: - derivate of rifamycin (Streptomyces) - inhibits initiation of RNA synthesis (binds to RNA polymerase -> in pocket where RNA-DNA hybrid is formed) Actinomycin D: - polypeptide-containing (Streptomyces) - binds tightly (intercalates) to ds-DNA (cannot be template for RNA synthesis) - its ability to inhibit growth of rapid dividing cells makes it a effective agent in cancer treatment 20 Transcription and Translation in Prokaryotes and Eukaryotes 21 α-Amanitin: produced by mushroom (Amanita phalloides) -> cyclic peptide of 8 amino acids -> binds tightly to RNA polymerase II -> blocks elongation of RNA synthesis -> deadly doses (LD50 is 0.1 mg/kg) 22 Different Eukaryotic RNA Polymerase promoters Inr -> Initiator element (found at transcription start) DPE -> downstream core promoter element Eukaryotic promoter elements (RNA polymerase II promoter) -> -40 and -150 Normally between -30 and -100 Often paired with Inr -> -3 and -5 CAAT boxes and GC boxes can even be on noncoding strand active DPE -> +28 and +32 24 Eukaryotic Transcription Initiation TappingMode AFM image of an individual human transcription factor 2: DNA complex. Clearly resolved are the protein:protein interactions of two transcription factor proteins which facilitate the looping of the DNA, allowing two distal DNA sites to be combined. AFM provided the investigators' improved resolution of the looped DNA complexes compared to electron microscopy of rotary shadowed samples. 252 nm scan. Image courtesy of Bustamante Lab, Institute of Molecular Biology, University of Oregon, Eugene. 25 Eukaryotic Transcription Initiation Basal transcription apparatus (-> carboxylterminal domain) TATA-box binding protein (TBP is a component of TFIID) recognizes the TATA box and forms complex with DNA CTD plays a role in transcrition regulation -> binds to mediator Phosphorylation of CTD by TFIIH -> elongation of transcription 26 Eukaryotic Transcription Initiation Complex 27 Regulation of Transcription 28 Packaging of Eukaryotic chromosomal DNA 29 Transcription Initiation 30 Gene “Off” Gene “On” 31 32 Eukaryotic transcription products (from RNA polymerase II) are processed triphosphate 7-methylguanylate end Capping 5’ end Polyadenylation of 3’ end 33 RNA editing 34 Splicing Anemia: defect synthesis of hemoglobin Mutations affecting splice sites cause Creates a new splice site around 15% of all genetic diseases 35 36 Small nuclear RNAs in spliceosomes catalyse the splicing 37 Spliceosome assembly The catalytic center of the spliceosome 38 Alternative splicing 39 Self-splicing A rRNA precursor of Tetrahymena (protozoan) splices itself in the presence of guanosine (G) as co-factor The L19 RNA is a intron that is catalytical active This TappingMode scan of the protozoan, Tetrahymena, shows its cilia-covered body and mouth structures. The sample was dried onto a glass slide and scanned; no other preparation was required. 50 micron scan courtesy C. Mosher and E. Henderson, BioForce Laboratory and Iowa State University. 40 Self-splicing mechanism 41 42 Ribosomal Factory Protein mRNA Translation 43 Translation: mRNA -> Protein 44 Peptide bond formation in Ribosomes 45 46 47 Linkage of Amino Acids to tRNA 2nd step 1st step Linkages either 2’ or 3’ 1st step: activation of AA by adenylation (Aminoacyl-AMP) 2nd step: linkage of AA to tRNA 48 Aminoacyl-tRNA synthetases couple Amino acids to tRNA Synthetases are highly specific for the amino acid (error rate 1 in 105) 49 50 Proofreading of Aminoacyl-tRNA Synthetases 51 Synthetases recognize the anticodon loops and acceptor stems of tRNA Threonyl-tRNA synthetase complex Class II synthetase Glutaminyl-tRNA synthetase complex Class I synthetase 52 Classification of Aminoacyl-tRNA synthetases Synthetases recognize different faces of the tRNA molecule: 1. Class I acylates the 2’ OH group of the terminal adenosine of tRNA 2. Class II acylates the 3’ OH group of the terminal adenosine of tRNA 3. They bind ATP in different conformations 4. Most class I are monomeric, most class II are dimers 53 54 Ribosomes are Ribonucleoproteins 50S 70S 30S 55 Ribosomal RNAs (5S, 16S, 23S rRNA) 16S rRNA tertiary structure secondary structure 56 Ribosomal Protein L19 of the 50S subunit Fits through some of the cavities within the 23S RNA 57 Protein synthesis in E. coli Polysomes: Transcription and Translation happens at the same time Direction of Transcription: 5’->3’ Direction of Translation: 5’->3’ 58 Translational Initiation sites – Ribosome binding sites 59 Bacterial Protein synthesis is initiated by Formylmethionyl tRNA -> fMet 60 tRNA binding sites on Ribosomes A for aminoacyl -> tRNA enters Ribosomes P for peptidyl -> tRNA passed on - peptide bonds are closed E for exit -> tRNA exits Ribosomes 61 Polypeptide chain escape path Polypeptide synthesis tunnel 62 63 Peptide bond formation 64 Some tRNAs recognize more than one codon -> wobble base 65 Elongation factor Tu EF-Tu delivers aminoacyltRNA to Ribosomes Elongation factor G EF-G mediates translocation within the Ribosome 66 Translocation mechanism EF-G (in GTP form) binds to EF-Tu site -> stimulates GTP hydrolysis Conformational change of EF-G -> driving EF-G into A site Causes translocation of tRNA and mRNA 67 Diphtheria Toxin blocks Protein Synthesis by Inhibition of Translocation Disease: Diphtheria Cause: Toxin from Corynebacterium diphtheriae Toxin catalysis transfer of ADP-ribose to diphthalamide ( a modified AA in EF 2 – translocase) 68 Protein Synthesis Termination by Release Factors 69 Differences between Eukaryotic and Prokaryotic Protein Synthesis Difference -> Translocation Initiation 70 Protein Interaction cirularize mRNA