activators - UCSF Tetrad Program
advertisement

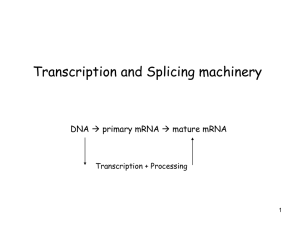
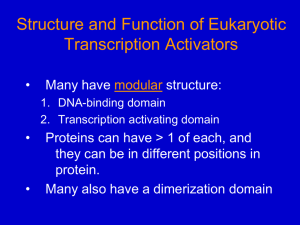
Biochemistry 201 Biological Regulatory Mechanisms: Lecture 3 January 28, 2013 Control of Transcription in Bacteria General References Chapter 16 of Molecular Biology of the Gene 6th Edition (2008) by Watson, JD, Baker, TA, Bell, SP, Gann, A, Levine, M, Losick, R. 547-587. Ptashne, M. and Gann, A. (2002) Genes and Signals. Cold Spring Harbor Laboratory Press, Cold Spring Harbor. Luscombe, N.M., Austin, S.E., Berman, H.M., Thornton, J.M. (2000) An overview of the structures of protein-DNA complexes. Genome Biology 1(1): reviews001.1-001.37 Examples of Control Mechanisms Alternative Sigma Factors Sorenson, MK, Ray, SS, Darst, SA (2004) Crystal structure of the flagellar sigma/anti-sigma complex 28 /FlgM reveals an intact sigma factor in an inactive conformation. Molecular Cell 14:127-138. Gruber, TM, Gross, CA (2003) Multiple sigma subunits and the partitioning of bacterial transcription space. Annu. Rev. Microbiol 57:441-66 Increasing the Initial Binding of RNA Polymerase Holoenzyme to DNA Lawson CL, Swigon D, Murakami KS, Darst SA, Berman HM, Ebright RH. (2004) Catabolite activator protein: DNA binding and transcription activation. Curr Opin Struct Biol. 14:10-20. Increasing the Rate of Isomerization of RNA Polymerase *Dove, S.L., Huang, F.W., and Hochschild, A. (2000) Mechanism for a transcriptional activator that works at the isomerization step. Proc Natl Acad Sci USA 97: 13215-13220. Jain, D. Nickels, B.E., Sun, L., Hochschild, A., and Darst, S.A. (2004) Structure of a ternary transcription activation complex. Mol Cell 13: 45-53. Hawley and McClure (1982) Mechanism of Activation of Transcription from the l PRM promoter. JMB 157: 493-525 DNA looping **Oehler, S., Eismann, E.R., Kramer, H. and Mueller-Hill, B. (1990) The three operators of the lac operon cooperate in repression. EMBO 9:973-979. Vilar, J.M.G. and Leibler, S. (2003) DNA looping and physical constraints on transcription regulation. J Mol Biol 331:981-989. Dodd, I.B., Shearwin, K.E., Perkins, A.J., Burr, T., Hochschild, A., and Egan, J.B. (2004) Cooperativity in long-range gene regulation by the l cI repressor. Genes Dev. 18:344-354. *Choi, PJ, Cai,L, Frieda K and X. Sunney Xie (2008) A Stochastic Single-Molecule Event Triggers Phenotype Switching of a Bacterial Cell Science 2008: 442-446. [DOI:10.1126/science.1161427] Attenuation/riboswitches Merino E and Yanofsky, C. (2005) Transcription attenuation: A highly conserved regulatory strategy used by bacteria. Trends in Genetics 21: 260 - 262 Winkler WC, Breaker RR. (2005) Regulation of bacterial gene expression by riboswitches. Annu Rev Microbiol 59:487-517. Landick R. (2009) Transcriptional pausing without backtracking. Proc Natl Acad Sci 106:8797-8. Serganov a and E. Nudler (2012) A decade of riboswitches. Cell 152: 17-24 (Review) Xia et al (2012): Riboswitch Control of Aminoglycoside Antibiotic resistance. Cell 152: 68 - 81 NusG and General Elongation Control Mooney, R………and Landick R. ( 2010) Two Structurally Independent Domains of E. coli NusG Create Regulatory Plasticity via Distinct Interactions with RNA Polymerase and Regulators. JMB 391: 341-351 Herbert, KM……Landick, R and Block, S. (2010) E. coli NusG Inhibits Backtracking and Accelerates Pause-Free Transcription by Promoting Forward Translocation of RNA Polymerase. JMB 399: 17 -30 Klein, B.,….and Murakami K. ( 2011). RNA polymerase and transcription elongation factor Spt4/5 complex structure. PNAS 108: 546-50 Coupling of translation and transcription Burmann, B…..Gottesman, M and Rosch, P. ( 2010) A NusE:NusG Complex Links Transcription and Translation Science 328: 501-4 *Proshkin, S..and Nudler, E. (2010). Cooperation Between Translating Ribosomes and RNA Polymerase in Transcription Elongation. Science 328: 504 -8 Important Points 1. Every step in transcription initiation can be regulated to increase or decrease the number of successful initiations per time. 2. In E. coli, transcription initiation is controlled primarily by alternative factors and by a large variety of other sequence-specific DNA-binding proteins. 3. G=RTlnKD. This means that a net increase of 1.4 kcal/mole (the approximate contribution of an additional hydrogen bond) increases binding affinity by 10-fold. Many examples of transcription activation in bacteria take advantage of such weak interactions. 4. To activate transcription at a given promoter by increasing KB, the concentration of RNA polymerase in the cell and its affinity for the promoter must be in the range so an increase in KB makes a difference. Likewise, to activate transcription by increasing kf, the rate of isomerization must be slow enough so the increase makes a substantial difference. 5. DNA looping allows proteins bound to distant sites on DNA to interact. 6. Transcriptional pausing and alternative RNA structures underlie many elongation control mechanisms. Control of Transcription in Bacteria Every step of transcription can be regulated NTPs KB R+P RPc initial binding Kf RPo “isomerization” Abortive Initiation Elongating Complex Gene regulation in E. coli: The Broad Perspective • 4400 genes • 300-350 sequence-specific DNA-binding proteins • 7 factors Alternative s are major control mechanism in bacteria Alternative s The Number of Sigma Factors Varies Dramatically among Bacteria Mycoplasma sp. Aquifex aeolicus Escherichia coli Bacillus subtilis Pseudomonas aeruginosa Streptomyces coelicolor 1 4 7 18 24 63 Alternative s direct RNAP to a discrete promoter set in response to a specific condition Figure 7–63 Interchangeable RNA polymerase subunits as a strategy to control gene expression in a bacterial virus. The bacterial virus SPO1, which infects the bacterium B. subtilis, uses the bacterial polymerase to transcribe its early genes immediately after the viral DNA enters the cell. One of the early genes, called 28, encodes a sigmalike factor that binds to RNA polymerase and displaces the bacterial sigma factor. This new form of polymerase specifically initiates transcription of the SPO1 “middle” genes. One of the middle genes encodes a second sigmalike factor, 34, that displaces the 28 product and directs RNA polymerase to transcribe the “late” genes.This last set of genes produces the proteins that package the virus chromosome into a virus coat and lyse the cell. By this strategy, sets of virus genes are expressed in the order in which they are needed; this ensures a rapid and efficient viral replication. From Molecular Biology of the Cell, 4th Edition. Tremendous Diversity Among the Minimal Sigma Class Regulation by repressors and activators (alter reactivity of 70-holoenzyme) A brief digression: How proteins recognize DNA All 4 bp can be distinguished in the major groove Common families of DNA binding proteins In vivo parameters for Sequence-Specific DNA binding proteins KD ≈ 10-6 - 10-10M in vivo In E. coli 1 copy/cell ≈ 10-9 M If KD = 10-9M and things are simple: 10 copies/cell 90% occupied 100 copies/cell 99% occupied I. Regulating transcription initiation at KB (initial binding) step Negative control: repressors (e.g. l, Lac ); prevent RNAP binding R -35 -10 Positive control: activators ( e.g. CAP); facilitate RNAP binding with favorable protein-protein contact Favorable contact A * RNAP holo -35 -10 Lac repressor and DNA looping Lac ~ 1980 -35 -10 Lac operator Lac 2000 O3 -90 O1 -35 -10 O2 +400 Oehler, 2000 O2 1/10 affinity of O1 O3 1/300 affinity of O1 What is the function of these weak operators? The weak operators significantly enhance represssion Oehler, 2000 Through DNA looping, Lac repressor binding to a “strong” operator (Om) can be helped by binding to a “weak” operator (OA) OK Om Oa Better! Om A mutant Lac repressor that cannot form tetramers is not helped by a weak site MM Theoretical consideration of effects of looping (2 operators) Representative states of the binding of the repressor to one operator (top panel) or to two operators (bottom panel). Om (main operator) binds repressor more tightly than Oa (auxiliary operator). Transcription takes place only in the states (i) and (iii), when Om is not occupied. The arrows indicate the possible transitions between states. Note that with one operator, a single unbinding event is enough for the repressor to completely leave the neighborhood of the main operator. With two operators, the repressor can escape from the neighborhood of the main operator only if it unbinds sequentially both operators. From: Vilar, J.M.G. and Leibler, S. (2003) DNA looping and physical constraints on transcription regulation. J Mol Biol 331:981-989 . I. Regulating transcription initiation at KB (initial binding) step Positive control: activators ( e.g. CAP); facilitate RNAP binding with favorable protein-protein contact Favorable contact A * RNAP holo -35 -10 ∆ G = RT lnKD; if * nets 1.4 kcal/mol, KB goes up 10-fold Activating by increasing KB is effective only if initial promoter occupancy is low If favorable contact nets 1.4Kcal/mole (KB goes up 10X) then: a) If initial occupancy of promoter is low RNAP A * RNAP 10% occupied 1% occupied Transcription rate increases 10-fold b) If initial occupancy of promoter is high RNAP 99% occupied A * RNAP 99.9% occupied Little or no effect on transcription rate A case study of activation at KB: CAP at the lac operon: How is CAP activated? cAMP Inactive CAP high glucose Active CAP Regulates >100 genes positively or negatively CAP at lac operon CAP increases transcription ~40-fold; KB ; no effect on kf Strategies to identify point of contact between CAP and RNAP 1. Isolate “positive control” (pc) mutations in CAP. These mutant proteins bind DNA normally but do not activate transcription M M 2. “Label transfer” (in vitro) from activator labeled near putative “pc” site to RNAP Activate X*; reduce S-S; X* is transferred to nearest site; determine location by protein cleavage studies; X* transferred to -CTD 3. Isolate CAP-non-responsive mutations in -CTD S-S-X* RNAP -35 -10 M RNAP -35 -10 II. Regulating transcription initiation at kf (isomerization) step KB R+P initial binding RPc Kf RPo Abortive Initiation Elongating Complex “isomerization” Case study: l repressor at PRM λcI binds cooperatively to operator sites OR1 and OR2 and interacts with to activate transcription from PRM KB kf 1/2 time O.C. formation __________________________________________________________________________________________________________________________________________ PRM PRM + lC1 at OR2 _ 107 M-1 7 X 10-4/sec 16 min 107 M-1 7 X 10-3/sec 1.6 min The interactions between lcI and are well established a) “pc” mutants in lcI b) “bypass mutants in Domain 4 rpo D Mutagenize rpoD plasmid Introduce into E. coli c) In an artificial construct, lcI “recruits” Domain 4 to the promoter d) Co-crystal of lcI and Domain 4 on promoter reveals expected contacts and no conformational changes Isolate mutants that restore activation by lpc Asp38 Asn38 Arg596 Why then does lcI function at kf His596 (post-recruitment) not at KB? Model for mechanism of action of λcI at PRM Activating region and its target (red patches) are misaligned in the closed complex but come into alignment subsequently during the process of open complex formation. Depicted in brackets is a hypothetical productive intermediate that is stabilized by λ cI. In the absence of lcI, formation of an unproductive intermediate limits open complex formation at PRM Dove S L et al. PNAS 2000;97:13215-13220 Attenuation control Promoting either elongation or termination by stabilizing alternative 2˚structures of mRNA Case study: ”Attenuation” at the trp operon Low Trp High Trp 1:2 is a pause hairpin 3:4 is an intrinsic terminator Leader peptide has tryptophan residues 2:3 is an “antiterminator” hairpin Regulated “attenuation” (termination) is widespread Switch between the “antitermination” and “termination” Stem-loop structures can be mediated by: 1. Ribosome pausing ( reflects level of a particular charged tRNA): regulates expression of amino acid biosynthetic operons in gram - bacteria 2. Uncharged tRNA: promotes anti-termination stem-loop in amino acyl tRNA synthetase genes in gm + bacteria 3. Proteins: stabilize either antitermination or termination stem-loop structures 4. Small molecules: aka riboswitches 5. Alternative 2˚ structures can also alter translation, self splicing, degradation General elongation control: NusG NusG-like NTD binds across the cleft in all three kingdoms of life, apparently locking the clamp against movements (& encircling DNA) adapted from Martinez-Rucobo et al. 2011 EMBO J. 30 NusG, the only universal elongation factor, exhibits divergent interactions with other regulators Case study: role of NusG: An essential elongation factor NTD CTD Activities: 1. Increases elongation rate 2. suppresses backtracking 3. Required for anti-termination mechanisms 4. Enhances termination mediated by the rho-factor How does one 21Kd protein mediate all of these activities? The NTD of NusG is sufficient to enhance elongation rate and to prevent backtracking! The NusG NTD interacts with RNAP (coiled coiled motif in b’) Current view of Pausing (?) Elemental Pause Elongation Complex The CTD of NusG interacts with other protein partners CTD 50 µM NusE Rho NusE is part of a complex of proteins mediating antitermination/termination depending on its protein partners Rho is an RNA binding hexamer that mediates termination by dissociating RNA from its complex with RNA polymerase and DNA using stepwise physical forces on the RNA derived from alternating protein conformations coupled to ATP hydrolysis Although the CTD mediates the protein interactions involved in termination and antitermination, full length NusG is required for both processes, presumably because NusG must be tethered to RNA polymerase for these functions NusG may also mediate ribosome/ RNAP interaction CTD 50 µM NusE NusE is ribosomal protein S10, and structural studies indicate that its binding site would be exposed when S10 is part of the ribosome. This protein protein interaction could connect these two major macromolecular machines Altering translation rate alters the transcription rate Condition + chloramphenicol ( 1µg/ml) Slow ribosome (streptomycin dependent) Slow ribosome (+ streptomycin) translation rate 14 aa/sec 9 aa/sec 6 aa/sec 10 aa/sec transcription rate 42 nt/sec 27 nt/sec 19 nt/sec 31 nt/sec Footprinting studies show that the presence of a ribosome behind RNA polymerase prevents backtracking! This could be a general mechanism to couple the rates of transcription and translation Coupled syntheses. J W Roberts Science 2010;328:436-437 Published by AAAS Single molecule experiments indicate that NusG suppresses backtracking, decreases the frequency of “elemental pause”, and modestly increases the pause-free elongation rate A unified pathway for elongation and pausing. The main pathway for transcript elongation is shown (light blue boxes; top row; adapted from Ref. 38). In a Brownian ratchet mechanism, RNAP oscillates stochastically between pre- and post-translocated states prior to the reversible binding of NTP followed by the (nearly) irreversible condensation reaction and pyrophosphate release, which rectify this motion in the transcriptionally downstream direction. The displacement associated with translocation, δ, corresponds to the longitudinal distance subtended by a single base pair. The elemental pause state is depicted (middle row, orange box; adapted from Ref. 14), shown branching from the pre-translocated state: entry into this state does not involve translocation. The long-lifetime, backtracked pause state (bottom row; orange box) is entered via the elemental pause state and involves the upstream translocation of RNAP through one or more base pairs, Nδ. Our modeling suggests that the addition of NusG promotes the downstream motion of RNAP, affecting those transitions that involve translocation (red arrows).