Activators bind to enhancer sites, controlled by hormones or other
advertisement
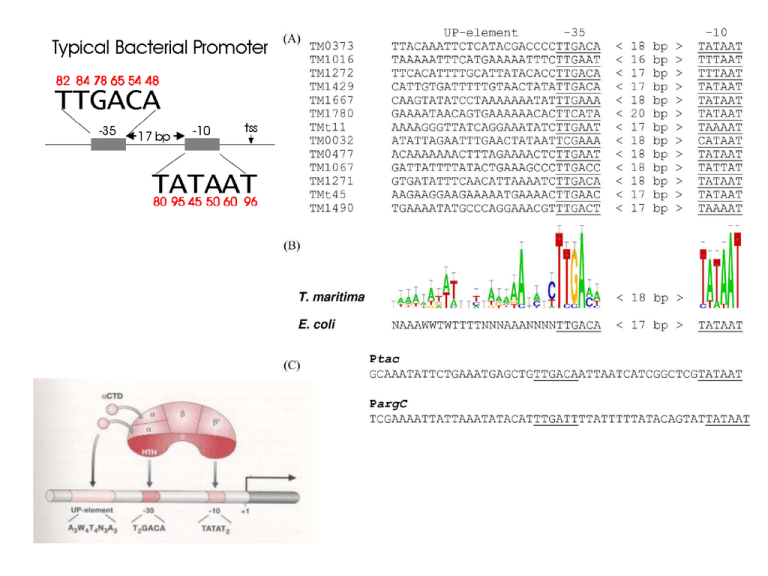
Activators bind to enhancer sites, controlled by hormones or other signals. They increase transcription of the regulated gene. Repressors bind to silencer sites, controlled by hormones or other signals. They decrease transcription of the regulated gene, possibly by interfering with activators. Coactivators bind to activators and/or repressors (at one end) and to basal factors (at the other end). The coactivators somehow communicate the signal from activators and/or repressors to the RNA polymerase. Basal factors act similar-ly to bacterial sigma factors. They enable RNA polymerase to initiate transcription. However, they require interaction with coactivators. + Comparison of a simple eukaryotic promoter and extensively diversified metazoan regulatory modules. a, Simple eukaryotic transcriptional unit. A simple core promoter (TATA), upstream activator sequence (UAS) and silencer element spaced within 100–200 bp of the TATA box that is typically found in unicellular eukaryotes. b, Complex metazoan transcriptional control modules. A complex arrangement of multiple clustered enhancer modules interspersed with silencer and insulator elements which can be located 10–50 kb either upstream or downstream of a composite core promoter containing TATA box (TATA), Initiator sequences (INR), and downstream promoter elements (DPE). Possible mechanisms for the regulation of genome expression by non-coding transcription. (A) Bidirectional PARs and mRNAs might originate from different preinitiation complexes (PICs) and compete for the same pool of transcription factors to initiate transcription. Binding of TBP or other factors might be responsible for directing the balance towards mRNA synthesis. (B) The transcriptional interference mechanism, in which transcription factors (TFs) are displaced from the mRNA promoter by the upstream cryptic transcription, is shown. The SRG1 cryptic noncoding RNA (ncRNA) interferes with the promoter of the downstream SER3 gene through this mechanism. (C) Model for start site selection. The CUT and the mRNA have the same promoter but originate from different transcription start sites and compete for the same pool of PIC factors. An example of this type of regulation occurs at the IMD2 locus. (D) Transcription-induced chromatin modifications, in which cryptic transcription modifies promoter proximal chromatin to attenuate gene expression. The GAL10–GAL1 locus is regulated through this mechanism; cryptic transcription that originates upstream from the GAL10–GAL1 promoter induces the methylation of H3K4 and/or H3K36 by the HMTs Set1 and Set2, respectively, and tethers the Rpd3S histone deacetylase complex to attenuate gene expression of the GAL locus. CUT, cryptic unstable transcript; H3, histone H3; HMT, histone methyl transferase; IMD2, inosine monophosphate dehydrogenase 2; K, lysine; PAR, promoter-associated non-coding RNA; Rpd3S, reduced potassium dependency 3 small; SER3, serine requiring 3; Set1/2, SET-domain-containing 1/2; SRG1, SER3 regulatory gene; TBP, TATA binding protein. Models for cis- or trans-mediated RNA-dependent regulation of gene expression. (A) Regulation in cis: when Rrp6 is delocalized or absent, the antisense CUT is stabilized and recruits HDACs, which are responsible for promoter regulation and silencing. This occurs, for example, at the PHO84 locus. (B) Regulation in trans: the CUT, which is transcribed from a distant locus and stabilized, induces the recruitment of the HMT Set1, thereby inhibiting gene transcription. The RTL non-coding RNA regulates the TY1 locus in this manner. CUT, cryptic unstable transcript; HDAC, histone deacetylase complex; HMT, histone methyl transferase; PHO84, phosphate metabolism 84; Rrp6, ribosomal RNA processing 6; RTL, antisense of LTR; Set1, SET-domain-containing 1; TY1, transposon in yeast 1. + A well-known example for Transcription termination is the in the tryptophan gene. The trp leader region can exist in alternative base-paired conformations: In the figure on the left the form created (two hairpins) is a transcription terminating. On the right the form created (one hairpin) is not terminating so the gene can be transcript. Slow intermediate mechanism. RNAP is depicted as in Fig. 1. At most template positions, fast translocation from the pretranslocated state to the active state allows tight NTP binding (assisted by a properly positioned 3′OH) and rapid transcription (top horizontal pathway)]. When RNAP encounters a pause, arrest, or termination site, it isomerizes to a slow intermediate in which the RNA 3′ end frays away from the DNA. A slight conformational opening of RNAP may precede and accelerate this change or may accompany it. Further rearrangement of the slow intermediate produces the different classes of paused, arrested, or terminating complexes. Escape of the slow intermediate back to the elongation pathway occurs by weak NTP binding and recapture of the 3′ OH in the active site. Amino acid substitutions in RNAP favor or disfavor the slow intermediate, whereas elongation factors NusA and NusG stabilize hairpinRNAP interaction or inhibit backtracking, respectively, at later steps in the pathway. Whether termination sometimes involves hairpin-RNAP interaction (dotted line) and whether it occurs via hairpin-induced bubble collapse or RNA pull-out (18, 36) remains to be determined. + Обща схема на репликация на ДНК Отрязване на интрони по механизма на “примката” (lariat) Структура на репликационната вилка Репликация на човешката ДНК Figure 4.20 Dideoxy DNA sequencing. Four separate reactions are performed, using the target sequence as a template. Each reaction contains a proportion of one nucleotide represented as a dideoxynucleotide. The dideoxynucleotide or the normal nucleotide can be incorporated into the growing strand, but incorporation of the dideoxynucleotide results in termination of DNA synthesis. Thus, each reaction results in a population of differently sized fragments that can be separated on a polyacrylamide gel and visualized by autoradiography. The sequence homologous to the template is read from bottom to top, 5‘ to 3‘, by noting the lane in which a band appears. Организация на генетичния материал в еукариотите All tRNAs possess a common secondary structure, the cloverleaf structure. The base sequence in the flattened model is for tRNA-Ala. Figure 11: Wobble may exist in the pairing of a codon on mRNA with an anticodon on tRNA. The mRNA and tRNA pair in an antiparallel fashion. Pairing at the first and second codon positions is in accord with the Watson and Crick pairing rules (A with U, G with C); however, pairing rules are relaxed at the third position of the codon, and G on the anticodon can pair with either U or C on the codon in this example. Алтернативен сплайсинг Alternative splicing results in different mature mRNAs and proteins. In mammals, the protein tropomyosin is encoded by a gene that has 11 exons. Tropomyosin pre-mRNA is spliced differently in different tissues, resulting in five different forms of the protein. Extensive variation in genome size within and among the main groups of life. Ever since the first general surveys of nuclear DNA content were carried out in the early 1950s, it has been apparent that eukaryotic genome sizes vary enormously and that this is unrelated to intuitive ideas of morphological complexity. This discrepancy between genome size and complexity remains clear more than half a century later, with genome sizes now available for nearly 9,000 species of animals and plants. In prokaryotes, genome size and gene number are strongly correlated, but in eukaryotes the vast majority of nuclear DNA is noncoding. Nevertheless, there is some overlap in genome size between the largest bacteria and the smallest parasitic protists. The figure illustrates the means and overall ranges of genome size that have been observed so far in the main groups of living organisms, and are loosely arranged according to common ideas of complexity to further emphasize the disparity between this parameter and genome size. Some commonly cited extreme values for amoebae (700,000 Mb) have been omitted, as there is considerable uncertainty about the accuracy of these measurements and the ploidy level of the species involved. Southern blotting and hybridization with probes can be used to locate a few specific fragments in a large pool of DNA. The current model for the biogenesis and post-transcriptional suppression of microRNAs and small interfering RNAs. The nascent pri-microRNA (primiRNA) transcripts are first processed into approximately 70nucleotide pre-miRNAs by Drosha inside the nucleus. Pre-miRNAs are transported to the cytoplasm by Exportin 5 and are processed into miRNA:miRNA* duplexes by Dicer. Dicer also processes long dsRNA molecules into small interfering RNA (siRNA) duplexes. Only one strand of the miRNA:miRNA* duplex or the siRNA duplex is preferentially assembled into the RNA-induced silencing complex (RISC) , which subsequently acts on its target by translational repression or mRNA cleavage, depending, at least in part, on the level of complementarity between the small RNA and its target. ORF, open reading frame. Gene regulation by RNA switches. RNA regions that are involved in gene expression switching are shown in the same color. a) Translation activation of virulence genes in the pathogen Listeria monocytogenes. An increase in temperature melts the secondary structure around the ribosome binding site (RBS) and start codon, allowing ribosome binding and translation initiation. b) Upregulation of an Escherichia coli gene by the DsrA antisense short RNA (sRNA). DsrA RNA pairs with the translational operator of the rpoS gene using two sequences (colored blue and light blue) located within helices 1 and 2. This base pairing exposes translation initiation signals for ribosome binding and increases mRNA stability. Eukaryotic cells have alternative pathways for processing pre-mRNA. (a) With alternative splicing; pre-mRNA can be spliced in different ways to produce different mRNAs. (b) With multiple 3 cleavage sites, there are two or more potential sites for cleavage and polyadenylation; use of the different sites produces mRNAs of different lengths. A transcription unit includes a promoter, an RNA-coding region, and a terminator. Transcription is initiated at RNA polymerase II promoters when the TFIID transcription factor binds to the TATA box, followed by the binding of a preassembled holoenzyme containing general transcription factors, RNA polymerase II, and the mediator. Steps 4 and 5 illustrate the binding of transcription activator proteins to the enhancer region, followed by the looping of DNA in order to allow the newly-formed structure to interact with the transcription apparatus. Аденовирус и вирус на грипа A transcription unit includes a promoter, an RNA-coding region, and a terminator.