Blackbody Radiation and Planck`s Hypothesis of Quantized Energy
advertisement

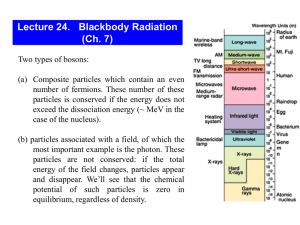
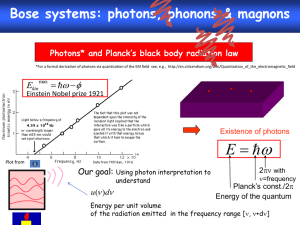
Knight - Chapter 28 (Grasshopper Book) Quantum Physics Blackbody Radiation and Planck’s Hypothesis of Quantized Energy An ideal blackbody absorbs all the light that is incident upon it. Blackbody Radiation and Planck’s Hypothesis of Quantized Energy An ideal blackbody is also an ideal radiator. If we measure the intensity of the electromagnetic radiation emitted by an ideal blackbody, we find: Blackbody Radiation and Planck’s Hypothesis of Quantized Energy This illustrates a remarkable experimental finding: The distribution of energy in blackbody radiation is independent of the material from which the blackbody is constructed — it depends only on the temperature, T. The peak frequency is given by: Blackbody Radiation and Planck’s Hypothesis of Quantized Energy The peak wavelength increases linearly with the temperature. This means that the temperature of a blackbody can be determined by its color. Classical physics calculations were completely unable to produce this temperature dependence, leading to something called the “ultraviolet catastrophe.” Blackbody Radiation and Planck’s Hypothesis of Quantized Energy Classical predictions were that the intensity increased rapidly with frequency, hence the ultraviolet catastrophe. http://www.astro.ubc.ca/~scharein/a311/Sim.html#Blackbody Blackbody Radiation and Planck’s Hypothesis of Quantized Energy Planck discovered that he could reproduce the experimental curve by assuming that the radiation in a blackbody came in quantized energy packets, depending on the frequency: The constant h in this equation is known as Planck’s constant: Blackbody Radiation and Planck’s Hypothesis of Quantized Energy Planck’s constant is a very tiny number; this means that the quantization of the energy of blackbody radiation is imperceptible in most macroscopic situations. It was, however, a most unsatisfactory solution, as it appeared to make no sense. *Photons and the Photoelectric Effect Einstein suggested that the quantization of light was real; that light came in small packets, now called photons, of energy: When a person visits the local tanning salon, they absorb photons of ultraviolet (UV) light to get the desired tan. What is the frequency and wavelength of a UV photon whose energy is 6.5x10-19 J? c 8 f f 3.00 10 m /s E h 9.80 10 14 310 nm 0.31 m Hz 6.5 10 6.63 10 19 34 J J s 9.8 10 14 Hz Photons and the Photoelectric Effect Therefore, a more intense beam of light will contain more photons, but the energy of each photon does not change. *Photons and the Photoelectric Effect The photoelectric effect occurs when a beam of light strikes a metal, and electrons are ejected. Each metal has a minimum amount of energy required to eject an electron, called the work function, W0. If the electron is given an energy E by the beam of light, its maximum kinetic energy is: Equation sheet- Kmax = hf - Φ Light of frequency 9.95x1014 Hz ejects electrons from the surface of silver. If the maximum kinetic energy of the ejected electrons is 0.180x10-19 what is the work function of silver? K m ax hf W 0 W 0 hf K m ax 6.63 10 = 6.42 10 19 J 34 J s 9.95 10 14 H z 0.180 10 19 J Photons and the Photoelectric Effect This diagram shows the basic layout of a photoelectric effect experiment. Photons and the Photoelectric Effect Classical predictions: 1. Any beam of light of any color can eject electrons if it is intense enough. 2. The maximum kinetic energy of an ejected electron should increase as the intensity increases. Observations: 1. Light must have a certain minimum frequency in order to eject electrons. 2. More intensity results in more electrons of the same energy. Photons and the Photoelectric Effect Explanations: 1. Each photon’s energy is determined by its frequency. If it is less than the work function, electrons will not be ejected, no matter how intense the beam. Photons and the Photoelectric Effect 2. A more intense beam means more photons, and therefore more ejected electrons. The Mass and Momentum of a Photon Photons always travel at the speed of light (of course!). What does this tell us about their mass and momentum? The total energy can be written: Since the left side of the equation must be zero for a photon, it follows that the right side must be zero as well. The Mass and Momentum of a Photon The momentum of a photon can be written: Dividing the momentum by the energy and substituting, we find: *The Mass and Momentum of a Photon Finally, we can write the momentum of a photon in the following way: What is the wavelength of a photon that has the same momentum as an electron moving with a speed of 1400 m/s? p m v 9.11 10 h p 31 kg 1400 m /s 1.275 10 6.63 10 1.275 10 34 27 J s kg m /s 27 kg m /s 520 nm 0.52 m Photon Scattering and the Compton Effect The Compton effect occurs when a photon scatters off an atomic electron. Photon Scattering and the Compton Effect In order for energy to be conserved, the energy of the scattered photon plus the energy of the electron must equal the energy of the incoming photon. This means the wavelength of the outgoing photon is longer than the wavelength of the incoming one: *The de Broglie Hypothesis and Wave-Particle Duality In 1923, de Broglie proposed that, as waves can exhibit particle-like behavior, particles should exhibit wave-like behavior as well. He proposed that the same relationship between wavelength and momentum should apply to massive particles as well as photons: What speed must a neutron have if its de Broglie wavelength is to be equal to the interionic spacing of table salt (0.282 nm)? h p v h m h mv 6.63 10 0.282 10 9 34 J s m 1.675 10 27 kg 1.40 km /s The de Broglie Hypothesis and Wave-Particle Duality The correctness of this assumption has been verified many times over. One way is by observing diffraction. We already know that Xrays can diffract from crystal planes: The de Broglie Hypothesis and Wave-Particle Duality The same patterns can be observed using either particles or X-rays. The de Broglie Hypothesis and Wave-Particle Duality Indeed, we can even perform Young’s twoslit experiment with particles of the appropriate wavelength and find the same diffraction pattern. The de Broglie Hypothesis and Wave-Particle Duality This is even true if we have a particle beam so weak that only one particle is present at a time – we still see the diffraction pattern produced by constructive and destructive interference. Also, as the diffraction pattern builds, we cannot predict where any particular particle will land, although we can predict the final appearance of the pattern. The de Broglie Hypothesis and Wave-Particle Duality These images show the gradual creation of an electron diffraction pattern.