File
advertisement

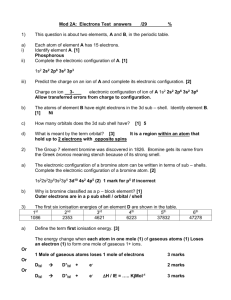
Ionization energy d. recall and understand the definition of ionization energies of gaseous atoms and that they are endothermic processes e. recall that ideas about electronic structure developed from: i. an understanding that successive ionization energies provide evidence for the existence of quantum shells and the group to which the element belongs ii. an understanding that the first ionization energy of successive elements provides evidence for electron sub-shells Connector: The mass spectrum for zirconium Given that the percentage abundance of its isotopes is: • zirconium-90 51.5 • zirconium-91 11.2 • zirconium-92 17.1 • zirconium-94 17.4 • zirconium-96 2.8 Calculate the relative atomic mass of zirconium Will Ionisation energy be Ionisation Energy ENDOTHERMIC OR EXOTHERMIC process? • It is the amount of energy required to remove a mole of electrons from a mole of gaseous atoms or ions. • Ionisation energy is measured in kJ/mole. • It can be represented as : The energy to remove the + _ first electron is called the • A (g) → A (g) + e first Ionisation energy • What will be the Ionisation energy equation for Sodium atom? + _ • Na (g) → Na (g) + e I.E. = 496 kJ/mol Ionisation Energy • In simple terms, Ionisation energy is the measure of how tightly or loosely the outer electron is attracted to the nucleus. • The less tightly it is bound, more reactive the element will be. • The Second Ionisation energy is the amount of energy required to remove a mole of electrons from a mole of gaseous cations (+ charged). + 2+ • Na (g) → Na (g) + e _ I.E. = 4563kJ/mol Comparison of first and second ionization energies of sodium First ionization energy of sodium Second ionization energy of sodium Notice the large difference in I.E. values. Suggest why this is so. Write an expression for the third ionization energy of sodium and predict its value. Atomic Line Emission Spectra and Niels Bohr Niels Bohr (1885-1962) • Bohr’s greatest contribution to science was in building a simple model of the atom. • If a gas is heated or an electric charge is passed through it, the gas gives out light. • If you pass the light through a prism, the light splits to give a spectrum. • The spectrum is made up of a series of separate lines or bands called Line spectrum or Emission spectrum. Spectrum of Excited Hydrogen Gas Bohr used the hydrogen spectrum to suggest that electrons are arranged in shells around the nucleus. Each shell represents a different energy level. Moving out from the centre of the atom, successive shells become closer together, in the same way as the energy levels in the line spectrum become closer. EXCITED STATE Energy transition in Hydrogen atom Energy GROUND STATE When ‘H’ atoms are heated, electrons are promoted from the GROUND STATE (low energy, highly stable) to EXCITED STATE (high energy, least stable) . Being UNSTABLE, electron drops back, giving out the energy gained in the form of LIGHT. Principal Quantum number (n) In an Atom, electrons are arranged in a series of shells. Each shell is described by a number called PRINCIPAL QUANTUM NUMBER (n). The larger the value of ‘n’, the further the electron will be from nucleus. Energy Levels n=1 n=2 n=3 n=4 Ionisation Ionisation energy energy increases increases remarkably for electron Aluminium – are Al13 are for 1,2electron and 3. Electrons 4 and 12. Electrons present in the Successive ionization energies Group 3 present same shell in the (n=3) different shells (n=1,2,3) The table below shows the successive ionisation energy (kJ/mole) of an atom. Numbers from 1 to 13 show the ionisation energy for the corresponding number of electrons 1 2 3 580 1820 2750 4 5 6 7 8 9 10 11 11600 14800 18400 23300 27500 31900 38500 42700 12 13 200 000 222 000 1. Name the atom and it’s group. 2. Explain the relative magnitude of the first, second and third ionisation energy. 3. Explain the relative magnitude of the first, fourth and twelfth ionisation energy 2.8.3 1 2 3 580 1820 2750 4 5 6 7 8 9 10 11 11600 14800 18400 23300 27500 31900 38500 42700 12 13 200 000 222 000 Line Spectra of Other Elements Unfortunately, this simple interpretation of line spectrum of Hydrogen did not explain the complex spectra of other elements. This lead to the suggestion that shells can be divided into sub-shells. Sub-shells • Each shell Arrange is divided all theinto subshells subshells. in anThese subshells increasing are described order ofby energy. letters: s,p,d,f. Shell Subshells 1 1s 2 3 4 2s, 2p 3s, 3p, 3d 4s, 4p, 4d, 4f Subshells have different energies. s (lowest energy)< p< d< f As the ‘n’ increases, the energy gap between successive shells decreases. As a result, neighbouring subshells overlap and have a different order of increasing energy in subshells. Diagonal Rule Steps: 1s 2s 3s 2p 3p 3d 1. Write the energy levels top to bottom. 2. Write the orbitals in s, p, d, f order. 3. Write the same number of orbitals as the energy level. 4. Draw diagonal lines from the top right to the bottom left. 5. To get the correct order, follow the arrows! 4s 4p 4d 4f 5s 5p 5d 5f 5g? 6s 6p 6d 6f 6g? 6h? 7s 7p 7d 7f 7g? 7h? By this point, we are past the current periodic table so we can stop. 7i? Electronic configuration Maximum number of electrons in ‘s’ can be 2 and ‘p’ can be 6. Write down the electronic configuration of Magnesium and Aluminium. Ionisation energy along a period increases as it is difficult to remove electrons from same shell with increasing effective nuclear charge. But there are some exceptions like B and Al. Look at their electronic configuration and explain the lowering of Ionisation energy. Ionization Energy: Periodic table Atomic number Ionisation Energy increases as the number of electrons removed increases (1) Large amount of energy difference in 1 & 2 indicate that these electrons are If an atom has one present in different shells. Similarly electron 9 and 10 are present in different valence electron eg Na, shell. (1) which group does it Atom has three shells.1 electron is to? present in outermost shell, 8 electrons in belong 2 2 6 1 the next shell and 2 electron in the innermost shell. 1s 2s 2p 3s (1) 3 marks Review of lesson Homework • Homework task: Complete question 1 and 2 on page 61 of Edexcel active book. • Due date: next lesson • Criteria for Grade C: • Criteria for Grade B: • Criteria for Grade A: