1. Structure of Matter
advertisement

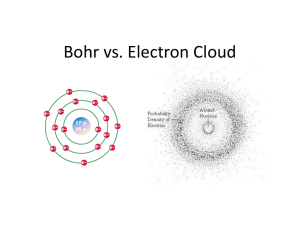
The structure of Matter Element , Compound, and Mixture compound compound Element Mixture : MgF2 in gasoline, water-gasoline, suger in water Element + compound 1 Compound 2 Chemical reaction Heterogeneous mixture Homogenous mixture No chemical reaction Persamaan reaksi kimia Zn + S C4H10 + 13 O2 ZnS 8 CO2 + 10 H2O Struktur atom 2500 tahun yang lalu : atom = “not cut” Dalton atomic teory: 1. Materi terdiri atas atom atom 2. Atom merupakan bagian terkecil dari materi dan tidak dapat terbelah lagi 3. Atom dari suatu element memiliki massa dan sifat sama 4. Atom dari element berbeda memiliki massa dan sifat berbeda 5. Atom atom dapat bergabung membentuk senyawa dengan rasio massa tertentu Bagaimana cara mengetahui massa atom saat periode teori atom Dalton? Struktur Atom modern Penemuan elektron • Menurut Dalton dan ilmuwan sebelumnya, atom tak terbagi dan merupakan komponen mikroskopik utama materi. Jadi, tidak ada seorangpun ilmuwan sebelum abad 19 menganggap atom memiliki struktur, atau dengan kata lain, atom juga memiliki komponen yang lebih kecil. • Keyakinan bahwa atom tak terbagi mulai goyah akibat perkembangan pengetahuan hubungan materi dan kelistrikan yang berkembang lebih lanjut. Kemajuan pemahaman hubungan materi dan listrik. • Faraday jumlah zat yang dihasilkan di elektroda-elektroda saat elektrolisis (perubahan kimia ketika arus listrik melewat larutan elektrolit) sebanding dengan jumlah arus listrik. Jumlah listrik yang diperlukan untuk menghasilkan 1 mol zat di elektroda adalah tetap (96,500 C).Hubungan ini dirangkumkan sebagai Hukum Elektrolisis Faraday. • Irish George Johnstone Stoney (1826-1911) memiliki wawasan sehingga mengenali pentingnya hukum Faraday pada struktur materi; ia menyimpulkan bahwa terdapat satuan dasar dalam elektrolisis, dengan kata lain ada analog atom untuk kelistrikan. Ia memberi nama elektron. • Berapa muatan 1 elektron? Percobaan tabung vakum Plücker (1801-1868). Bila kation mengenai anoda dan diberikan beda potensial yang tinggi pada tekanan rendah (lebih rendah dari 10–2 - 10–4 Torr), gas dalam tabung, walaupun merupakan insulator, menjadi penghantar dan memancarkan cahaya. Bila vakumnya ditingkatkan, dindingnya mulai menjadi mengkilap, memancarkan cahaya fluoresensi Beberapa partikel dipancarkan dari katoda. Ia memberi nama sinar katoda pada partikel yang belum teridentifikasi ini • Fisikawan Inggris Joseph John Thomson (1856-1940) menunjukkan bahwa partikel ini bermuatan negatif. • Fisikawan Amerika Robert Andrew Millikan (1868-1953) berhasil membuktikan dengan percobaan yang cerdas adanya partikel kelistrikan ini. Percobaan yang disebut dengan percobaan tetes minyak Millikan. Tetesan minyak dalam tabung jatuh akibat pengaruh gravitasi. Bila tetesan minyak memiliki muatan listrik, gerakannya dapat diatur dengan melawan gravitasi dengan diberikan medan listrik. Gerakan gabungan ini dapat dianalisis dengan fisika klasik. Millikan menunjukkan dengan percobaan ini bahwa muatan tetesan minyak (elektron) selalu merupaka kelipatan 1,6x10–19 C. Muatan elektron =1,6 x 10–19 C. Ratio muatan/massa (Thomson ) = 1,76 x108 C/g), • Latihan 1. Perhitungan massa elektron. Hitung massa elektron dengan menggunakan nilai yang didapat Millikan dan Thomson. Jawab: Anda dapat memperoleh penyelesaian dengan mensubstitusikan nilai yang didapat Millikan pada hubungan: muatan/massa = 1,76 x 108 (C g–1). Maka, m = e/(1,76 x 108 C g–1) = 1,6 x 10–19 C/(1,76 x 108C g–1) = 9,1 x 10–28 g. Muatan elektron =1,6 x 10–19 C. Ratio muatan/massa (Thomson ) = 1,76 x108 C/g), Latihan 2. Rasio massa elektron dan atom hidrogen. Hitung rasio massa elektron dan atom hidrogen! Jawab: Massa mH atom hidrogen atom adalah: mH = 1 g/6 x 1023 = 1,67 x 10–24g. Jadi, me : mH = 9,1 x 10–28g : 1,67 x10–24g = 1 : 1,83 x 103. Sangat menakjubkan bahwa massa elektron sangat kecil. Bahkan atom yang paling ringanpun, hidrogen, sekitar 2000 kali lebih berat dari massa elektron. Model atom Mengukur ukuran atom Latihan 3. Volume satu molekul air Dengan menganggap molekul air berbentuk kubus, hitung panjang sisi kubusnya. Dengan menggunakan nilai yang didapat, perkirakan ukuran kira-kira satu atom (nyatakan dengan notasi saintifik 10x). Jawab: Volume 1 mol air sekitra 18 cm3. Jadi volume 1 molekul air: v = 18 cm3/6 x 1023 = 3x10–23 cm3 = 30 x 10–24 cm3. Panjang sisi kubus adalah (30 x 10–24)1/3 cm = 3,1 x 10–8 cm. Nilai ini mengindikasikan bahwa ukuran atom sekitar 10–8 cm. Thomson mengasumsikan bahwa atom dengan dimensi sebesar itu adalah bola seragam bermuatan positif dan elektron-elektron kecil yang bermuatan negatif tersebar di bola tersebut. Dalam kaitan ini model Thomson sering disebut dengan “model bolu kismis”, kismisnya seolah elektron dan bolunya adalah atom. • Apa kelemahan model atom Thomson? - tidak dapat menerangkan susunan muatan positif / negatif dalam sebuah atom. Teori atom Bohr • The demonstration by Thompson in 1867 that all atoms contain units of negative electric charge led to the first science-based model of the atom which envisaged the electrons being spread out uniformly throughout the spherical volume of the atom. Ernest Rutherford, a New Zealander who started out as Thompson's student at Cambridge, distrusted this "plum pudding" model (as he called it) and soon put it to rest; Rutherford's famous alpha-ray bombardment experiment (carried out, in 1909, by his students Hans Geiger and Ernest Marsden) showed that nearly all the mass of the atom is concentrated in an extremely small (and thus extremely dense) body called the nucleus. This led him to suggest the planetary model of the atom, in which the electrons revolve in orbits around the nuclear "sun". Menurut ide Rutherford, muatan positif atom terpusat di bagian pusat (dengan jarijari terhitung sekitar 10–12 cm) sementara muatan negatifnya terdispersi di seluruh ruang atom. Partikel kecil di pusat ini disebut dengan inti. Semua model atom sebelumnya sebagai ruang yang seragam dengan demikian ditolak. • Namun, model atom Rutherford yang terdiri atas inti kecil dengan elektron terdispersi di sekitarnya tidak dapat menjelaskan semua fenomena yang dikenal. Bila elektron tidak bergerak, elektron akan bersatu dengan inti karena tarikan elektrostatik (gaya Coulomb). Ha ini jeals tidak mungkin terjadi sebab atom adalah kesatuan yang stabil. Bila elektron mengelilingi inti seperti planet dalam pengaruh gravitasi matahari, elektron akan mengalami percepatan dan akan kehilangan energi melalui radiasi elektromagnetik. Akibatnya, orbitnya akan semakin dekat ke inti dan akhirnya elektron akan jatuh ke inti. Dengan demikian, atom akan memancarkan spektrum yang kontinyu. Tetapi faktanya, atom yang stabil dan diketahui atom memancarkan spektrum garis bukan spektrum kontinyu. Jelas diperlukan perubahan fundamenatal dalam pemikiran untuk menjelaskan semua fakta-fakta percobaan ini. Dasar-dasar teori kuantum klasik Spektrum atom Bila gas ada dalam tabung vakum, dan diberi beda potensial tinggi, gas akan terlucuti dan memancarkan cahaya. Pemisahan cahaya yang dihasilkan dengan prisma akan menghasilkan garis spektra garis diskontinyu. Karena panjang gelombang cahaya khas bagi atom, spektrum ini disebut dengan spektrum atom. Fisikawan Swedia Johannes Robert Rydberg (1854-1919) menemukan bahwa bilangan gelombang7 σ garis spektra dapat diungkapkan dengan persamaan berikut (1889) ni dan nj bilangan positif bulat(ni < nj) dan R adalah tetapan khas untuk gas yang digunakan. Untuk hidrogen R bernilai 1,09678 x 107 m–1. Umumnya bilangan gelombang garis spektra atom hodrogen dapat diungkapkan sebagai perbedaan dua suku R/n2. Spektra atom gas lain jauh lebih rumit, tetapi sekali lagi bilangan gelombangnya juga dapat diungkapkan sebagai perbedaan dua suku. Model atom Bohr • Bohr suggested that the planetary model could be saved if one new assumption were made: certain "special states of motion" of the electron, corresponding to different orbital radii, would not result in radiation, and could therefore persist indefinitely without the electron falling into the nucleus. Specifically, Bohr postulated that the angular momentum of the electron, mvr (the mass and angular velocity of the electron and in an orbit of radius r) is restricted to values that are integral multiples of h/2π. The radius of one of these allowed Bohr orbits is given by • in which h is Planck's constant, m is the mass of the electron, v is the orbital velocity, and n can have only the integer values 1, 2, 3, etc. The most revolutionary aspect of this assumption was its use of the variable integer n; this was the first application of the concept of the quantum number to matter. The larger the value of n, the larger the radius of the electron orbit, and the greater the potential energy of the electron. • As the electron moves to orbits of increasing radius, it does so in opposition to the restoring force due to the positive nucleus, and its potential energy is thereby raised. This is entirely analogous to the increase in potential energy that occurs when any mechanical system moves against a restoring force— as, for example, when a rubber band is stretched or a weight is lifted. • Thus what Bohr was saying, in effect, is that the atom can exist only in certain discrete energy states: the energy of the atom is quantized. Bohr noted that this quantization nicely explained the observed emission spectrum of the hydrogen atom. The electron is normally in its smallest allowed orbit, corresponding to n = 1; upon excitation in an electrical discharge or by ultraviolet light, the atom absorbs energy and the electron gets promoted to higher quantum levels. These higher excited states of the atom are unstable, so after a very short time (around 10—9 sec) the electron falls into lower orbits and finally into the innermost one, which corresponds to the atom's ground state. The energy lost on each jump is given off as a photon, and the frequency of this light provides a direct experimental measurement of the difference in the energies of the two states, according to the Planck-Einstein relationship e = hν. How the Bohr model explains the hydrogen line spectrum? • Each spectral line represents an energy difference between two possible states of the atom. Each of these states corresponds to the electron in the hydrogen atom being in an "orbit" whose radius increases with the quantum number n. The lowest allowed value of n is 1; because the electron is as close to the nucleus as it can get, the energy of the system has its minimum (most negative) value. This is the "normal" (most stable) state of the hydrogen atom, and is called the ground state. •If a hydrogen atom absorbs radiation whose energy corresponds to the difference between that of n=1 and some higher value of n, the atom is said to be in an excited state. Excited states are unstable and quickly decay to the ground state, but not always in a single step. For example, if the electron is initially promoted to the n=3 state, it can decay either to the ground state or to the n=2 state, which then decays to n=1. Thus this single n=1→3 excitation can result in the three emission lines depicted in the diagram above, corresponding to n=3→1, n=3→2, and n=2→1. • If, instead, enough energy is supplied to the atom to completely remove the electron, we end up with a hydrogen ion and an electron. When these two particles recombine (H+ + e– → H), the electron can initially find itself in a state corresponding to any value of n, leading to the emission of many lines. • The lines of the hydrogen spectrum can be organized into different series according to the value of n at which the emission terminates (or at which absorption originates.) The first few series are named after their discoverers. The most well-known (and first-observed) of these is the Balmer series, which lies mostly in the visible region of the spectrum. The Lyman lines are in the ultraviolet, while the other series lie in the infrared. The lines in each series crowd together as they converge toward the series limit which corresponds to ionization of the atom and is observed as the beginning of the continuum emission. Note that the ionization energy of hydrogen (from its ground state) is 1312 kJ mol–1. • Although an infinite number of n-values are possible, the number of observable lines is limited by our ability to resolve them as they converge into the continuum; this number is around a thousand. Contoh soal: Jari-jari r dapat diungkapan dalam persamaan r = n2aB, n = 1, 2, 3,... aB adalah jari-jari minimum (jari-jari Bohr= 5,2918 x 10– 11 m) bila n = 1. Tentukan jari-jari lainnya untuk n=2,3,4 Hukum Moseley Henry Gwyn Jeffreys Moseley (1887-1915) mendapatkan, dengan menembakkan elektron berkecepatan tinggi pada anoda logam, bahwa frekuensi sinar-X yang dipancarkan khas bahan anodanya. Panjang gelombang λ sinar- X berkaitan dengan muatan listrik Z inti. Menurut Moseley, terdapat hubungan antara dua nilai ini (hukum Moseley; 1912). c dan s adalah tetapan yang berlaku untuk semua unsur, dan Z adalah bilangan bulat. Contoh: Perkiraan nomor atom dengan hukum Moseley Didapatkan bahwa sinar-X khas unsur yang tidak diketahui adalah 0,14299 x 10–9 m. Panjang gelombang dari deret yang sama sinar-X khas unsur Ir (Z = 77) adalah 0,13485 x 10–9 m. Dengan asumsi s = 7,4, perkirakan nomor atom unsur yang tidak diketahui tersebut. Jawab: Pertama perkirakan √c dari persamaan : [1/0,13485x10−9 (m)]1/2 = √ c. (77 − 7.4) = 69,6 √c; jadi √c = 1237,27, maka [1/0,14299x10−9 (m)] = 1237 (z − 7.4) dan didapat z = 75 Keterbatasan teori Bohr Keberhasilan teori Bohr begitu menakjubkan. Teori Bohr dengan sangat baik menggambarkan struktur atom hidrogen, dengan elektron berotasi mengelilingi inti dalam orbit melingkar.. Setelah berbagai penyempurnaan, teori Bohr mampu menerangkankan spektrum atom mirip hidrogen dengan satu elektron seperti ion helium He+. Namun, spektra atom atom poli-elektronik tidak dapat dijelaskan. Kemudian menjadi jelas bahwa ada keterbatasan dalam teori ini yaitu tidak ada penjelasan persuasif tentang ikatan kimia dapat diperoleh. Dengan kata lain,teori Bohr adalah satu langkah ke arah teori struktur atom yang dapat berlaku bagi semua atom dan ikatan kimia. Pentingnya teori Bohr tidak dapat diremehkan karena teori ini dengan jelas menunjukkan pentingnya teori kuantum untuk memahami struktur atom, dan secara lebih umum struktur materi. Question 1. 2. 3. 4. 5. 6. 7. Describe the Thompson, Rutherford, and early planetary models of the atom, and explain why the latter is not consistent with classical physics. State the major concepts that distinguished Bohr's model of the atom from the earlier planetary model. Give an example of a mechanical standing wave; state the meaning and importance of its boundary conditions. Sketch out a diagram showing how the concept of a standing wave applies to the description of the electron in a hydrogen atom. What is an atomic line emission spectrum? What is the significance of the continuum region of an emission spectrum? Sketch out a drawing showing the essentials of such a spectrum, including the ionization limit and the continuum. Describe the way in which Bohr's quantum numbers explain the observed spectrum of a typical atom. Explain the relation between the absorption and emission spectrum of an atom. • About ten years after Bohr had developed his theory, de Broglie showed that the electron should have wavelike properties of its own, thus making the analogy with the mechanical theory of standing waves somewhat less artificial. One serious difficulty with the Bohr model still remained, however: it was unable to explain the spectrum of any atom more complicated than hydrogen. A refinement suggested by Sommerfeld assumed that some of the orbits are elliptical instead of circular, and invoked a second quantum number, l, that indicated the degree of ellipticity. This concept proved useful, and it also began to offer some correlation with the placement of the elements in the periodic table. The Schrödinger equation Of these alternative treatments, the one developed by Schrödinger is the most easily visualized. Schrödinger started with the simple requirement that the total energy of the electron is the sum of its kinetic and potential energies. • The second term represents the potential energy of an electron (whose charge is denoted by e) at a distance r from a proton (the nucleus of the hydrogen atom). In quantum mechanics it is generally easier to deal with equations that use momentum (p = mv) rather than velocity, so the next step is to make this substitution: This is still an entirely classical relation, as valid for the waves on a guitar string as for those of the electron in a hydrogen atom. The third step is the big one: in order to take into account the wavelike character of the hydrogen atom, a mathematical expression that describes the position and momentum of the electron at all points in space is applied to both sides of the equation. The function, denoted by Ψ (psi), "modulates" the equation of motion of the electron so as to reflect the fact that the electron manifests itself with greater probability in some locations that at others. This yields the celebrated Schrödinger equation Why doesn't the electron fall into the nucleus? • We can now return to the question which Bohr was unable to answer in 1912. Even the subsequent discovery of the wavelike nature of the electron and the analogy with standing waves in mechanical systems did not really answer the question; the electron is still a particle having a negative charge and is attracted to the nucleus. • The answer comes from the Heisenberg uncertainty principle, which says that a quantum particle such as the electron cannot simultaneously have sharply-defined values of location and of momentum (and thus kinetic energy). To understand the implications of this restriction, suppose that we place the electron in a small box. The walls of the box define the precision δx to which the location is known; the smaller the box, the more exactly will we know the location of the electron. But as the box gets smaller, the uncertainty in the electron's kinetic energy will increase. As a consequence of this uncertainty, the electron will at times possess so much kinetic energy (the "confinement energy") that it may be able to penetrate the wall and escape the confines of the box. • The region near the nucleus can be thought of as an extremely small funnel-shaped box, the walls of which correspond to the electrostatic attraction that must be overcome if an electron confined within this region is to, the volume to which it is confined diminishes rapidly. Because its location is now more precisely known escape. As an electron is drawn toward the nucleus by electrostatic attraction, its kinetic energy must become more uncertain; the electron's kinetic energy rises more rapidly than its potential energy falls, so that it gets ejected back into one of its allowed values of n. • We can also dispose of the question of why the orbiting electron does not radiate its kinetic energy away as it revolves around the nucleus? The Schrödinger equation completely discards any concept of a definite path or trajectory of a particle; what was formerly known as an "orbit" is now an "orbital", defined as the locations in space at which the probability of finding the electrons exceeds some arbitrary value. It should be noted that this wavelike character of the electron coexists with its possession of a momentum, and thus of an effective velocity, even though its motion does not imply the existence of a definite path or trajectory that we associate with a more massive particle. Kelahiran mekanika kuantum De Broglie (1892-1987) P = mv Prinsip ketidakpastian Heisenberg Persamaan Schrödinger Persamaan Schrödinger BILANGAN KUANTUM The quantum numbers • Modern quantum theory tells us that the various allowed states of existence of the electron in the hydrogen atom correspond to different standing wave patterns. In the preceding lesson we showed examples of standing waves that occur on a vibrating guitar string. The wave patterns of electrons in an atom are different in two important ways: • Instead of indicating displacement of a point on a vibrating string, the electron waves represent the probability that an electron will manifest itself (appear to be located) at any particular point in space. (Note carefully that this is not the same as saying that "the electron is smeared out in space"; at any given instant in time, it is either at a given point or it is not.) • The electron waves occupy all three dimensions of space, whereas guitar strings vibrate in only two dimensions. • Aside from this, the similarities are striking. Each wave pattern is identified by an integer number n, which in the case of the atom is known as the principal quantum number. The value of n tells how many peaks of amplitude (antinodes) exist in that particular standing wave pattern; the more peaks there are, the higher the energy of the state. The three simplest orbitals of the hydrogen atom are depicted above in pseudo3D, in cross-section, and as plots of probability (of finding the electron) as a function of distance from the nucleus. The average radius of the electron probability is shown by the blue circles or plots in the two columns on the right. These radii correspond exactly to those predicted by the Bohr model Physical significance of n • The potential energy of the electron is given by the formula in which e is the charge of the electron, m is its mass, h is Planck's constant, and n is the principal quantum number. The negative sign ensures that the potential energy is always negative. Notice that this energy in inversely proportional to the square of n, so that the energy rises toward zero as n becomes very large, but it can never exceed zero. This formula was actually part of Bohr's original theory, and is still applicable to the hydrogen atom, although not to atoms containing two or more electrons. In the Bohr model, each value of n corresponded to an orbit of a different radius. The larger the orbital radius, the higher the potential energy of the electron; the inverse square relationship between electrostatic potential energy and distance is reflected in the inverse square relation between the energy and n in the above formula. Although the concept of a definite trajectory or orbit of the electron is no longer tenable, the same orbital radii that relate to the different values of n in Bohr's theory now have a new significance: they give the average distance of the electron from the nucleus. The angular momentum quantum number • The electron wave functions that are derived from Schrödinger's theory are characterized by several quantum numbers. The first one, n, describes the nodal behavior of the probability distribution of the electron, and correlates with its potential energy and average distance from the nucleus as we have just described. • The theory also predicts that orbital having the same value of n can differ in shape and in their orientation in space. The quantum number l, known as the angular momentum quantum number, determines the shape of the orbital. (More precisely, l determines the number of angular nodes, that is, the number of regions of zero probability encountered in a 360° rotation around the center.) • When l = 0, the orbital is spherical in shape. If l = 1, the orbital is elongated into something resembling, and higher values of l correspond to still more complicated shapes— but note that the number of peaks in the radial probability distributions (below) decreases with increasing l. The possible values that l can take are strictly limited by the value of the principal quantum number; l can be no greater than n – 1. This means that for n = 1, l can only have the single value zero which corresponds to a spherical orbital. For historical reasons, the orbitals corresponding to different values of l are designated by letters, starting with s for l = 0, p for l = 1, d for l = 2, and f for l = 3. The shapes and radial distributions of the orbitals corresponding to the three allowed values of l for the n = 3 level of hydrogen are shown above. Notice that the average orbital radius r decreases somewhat at higher values of l. The function relationship is given by in which z is the nuclear charge of the atom, which of course is unity for hydrogen. The magnetic quantum number m • An s-orbital, corresponding to l = 0, is spherical in shape and therefore has no special directional properties. The probability cloud of a p orbital is aligned principally along an axis extending along any of the three directions of space. The additional quantum number m is required to specify the particular direction along which the orbital is aligned. • The quantum number m can assume 2l + 1 values for each value of l, from –l through 0 to +l. When l = 0 the only possible value of m will also be zero, and for the p orbital (l = 1), m can be –1, 0, and +1. Higher values of l introduce more complicated orbital shapes which give rise to more possible orientations in space, and thus to more values of m. Electron spin and the exclusion principle • Certain fundamental particles have associated with them a magnetic moment that can align itself in either of two directions with respect to an external magnetic field. The electron is one such particle, and the direction of its magnetic moment is called its spin. • Electron spin is basically a relativistic effect in which the electron's momentum distorts local space and time. It has no classical counterpart and thus cannot be visualized other than through mathematics. • A basic principle of modern physics states that for particles such as electrons that possess half-integral values of spin, no two of them can be in identical quantum states within the same system. The quantum state of a particle is defined by the values of its quantum numbers, so what this means is that no two electrons in the same atom can have the same set of quantum numbers. This is known as the Pauli exclusion principle, named after the German physicist Wolfgang Pauli (1900-1958, Nobel Prize 1945). • The exclusion principle was discovered empirically and was placed on a firm theoretical foundation by Pauli in 1925. A complete explanation requires some familiarity with quantum mechanics, so all we will say here is that if two electrons possess the same quantum numbers n, l, m and s (defined below), the wave function that describes the state of existence of the two electrons together becomes zero, which means that this is an "impossible" situation. • A given orbital is characterized by a fixed set of the quantum numbers n, l, and m. The electron spin itself constitutes a fourth quantum number s, which can take the two values +1 and –1. Thus a given orbital can contain two electrons having opposite spins, which "cancel out" to produce zero magnetic moment. Two such electrons in a single orbital are often referred to as an electron pair. • Since the quantum numbers m and l are zero for n=1, the pair of electrons in the helium orbital have the values (n, l, m, s) = (1,0,0,+1) and (1,0,0,–1)— that is, they differ only in spin. These two sets of quantum numbers are the only ones that are possible for a n=1 orbital. The additional electrons in atoms beyond helium must go into higherenergy (n>1) orbitals. Electron wave patterns corresponding to these greater values of n are concentrated farther from the nucleus, with the result that these electrons are less tightly bound to the atom and are more accessible to interaction with the electrons of neighboring atoms, thus influencing their chemical behavior. If it were not for the Pauli principle, all the electrons of every element would be in the lowest-energy n=1 state, and the differences in the chemical behavior the different elements would be minimal. Summary: p orbitals and d orbitals p orbitals look like a dumbell with 3 orientations: px, py, pz (“p sub z”). Four of the d orbitals resemble two dumbells in a clover shape. The last d orbital resembles a p orbital with a donut wrapped around the middle. Make sure you thoroughly understand the following essential ideas which have been presented above. It is especially imortant that you know the precise meanings of all the highlighted terms in the context of this topic. 1. State the fundamental distinction between Bohr's original model of the atom and the modern orbital model. 2. Explain the role of the uncertainty principle in preventing the electron from falling into the nucleus. 3. State the physical meaning of the principal quantum number of an electron orbital, and make a rough sketch of the shape of the probability-vs-distance curve for any value of n. 4. Sketch out the shapes of an s, p, or a typical d orbital. 5. Describe the significance of the magnetic quantum number as it applies to a p orbital. 6. State the Pauli exclusion principle. Order of sublevel energies: s<p<d<f The Quantum-Mechanical Model and The Periodic Table • A useful way to determine the electron configuration of the elements is to add one electron per element to the lowest energy orbital available. This approach called the aufbau principle (German aufbauen, “to build up”) • It results in ground state electron configuration. • There are two common ways to show the orbital occupancy: – The electron configuration – The orbital diagram Building up periods 1 and 2 • The placement of electrons for carbon exemplifies Hund’s Rule: when orbitals of equal energy are available the electron configuration of lowest energy has the maximum number of unpaired electrons with parallel spins Sample Problems • Write a set of quantum numbers for the third and eighth electrons added to F • Use the periodic table to identify the element with the electron configuration 1s22s22p4. Write its orbital diagram and give the quantum numbers of its sixth electron. Answers • The third electron is in the 2s orbital. n = 2. l = 0, ml = 0, ms = + ½ • The eighth electron is in the first 2p orbital n = 2, l = 1, ml = -1, ms = - ½ • The element has eight electron so Z = 8 oxygen 1s 2s 2p n = 2, l = 1, ml = 0, ms = + ½ Relation between orbital filling and the periodic table Categories of electrons • Inner (core) electrons are those in the previous noble gas and any completed transition series. They fill all the lower energy levels of an atom • Outer electron are those in the highest energy level (highest n value). They spent most of their time farthest from nucleus • Valence electron are those involved in forming compounds. Among the main group elements, the valence electrons are the outer electrons. Among the transition elements, some inner d electrons are also often involved in bonding and are counted among the valence electrons. Sample Problems • • Give the (1) full and condensed electron configurations, (2) partial orbital diagrams for the valence electrons and (3) number of inner electrons for the following element: 1. Potassium (K: Z = 19) 2. Molybdenum (Mo: Z = 42) 3. Lead (Pb: Z = 82) Give full and condensed electron configurations, a partial diagrams for valence electrons and the number of inner electrons for the following element: 1. Ni (Z = 28) 2. Sr (Z = 38) 3. Po (Z = 84) Answers • K (Z = 19) Full: 1s2 2s2 2p6 3s2 3p6 4s1 Condensed: [Ar] 4s1 • Mo (Z = 42) Full: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s1 4d5 Condensed : [Kr] 5s1 4d5 • Pb (Z = 82) Full: 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s2 4d10 5p6 6s2 4f14 5d10 6p2 Condensed: [Xe] 6s2 4f14 5d10 6p2 Trends in Atomic Size • Size goes UP on going down a group. • Because electrons are added further from the nucleus, there is less attraction. • Size goes DOWN on going across a period. Atomic Radii Atomic Size • Size decreases across a period owing to increase in Z*. • Each added electron feels a greater and greater + charge. Na Mg Al Si P S Cl Ar 186 160 143 118 110 103 100 98 Sample Problem • Using only the periodic table, rank each set of main-group elements in order of decreasing atomic size: 1. 2. 3. 4. Ca, Mg, Sr K, Ga, Ca Br, Rb, Kr Sr, Ca, Rb 1. Sr > Ca > Mg 2. K > Ca > Ga 3. Rb > Br > Kr 4. Rb > Sr > Ca Ion Sizes • CATIONS are SMALLER than the atoms from which they come. • The electron/proton attraction has gone UP and so size DECREASES. • 3- > 2- > 1- > 1+ > 2+ > 3+ Ion Sizes • ANIONS are LARGER than the atoms from which they come. • The electron/proton attraction has gone DOWN and so size INCREASES. • Trends in ion sizes are the same as atom sizes. Sampel problems • Rank the ions in order of increasing size • Na+, Mg2+, F• Ca2+, Sr2+, Mg2+ Answers : • Mg2+< Na+ < F• Mg2+ < Ca2+ < Sr2+ Ionization Energy • IE = energy required to remove an electron from an atom in the gas phase. Mg (g) + 735 kJ ---> Mg+ (g) + eMg+ (g) + 1451 kJ ---> Mg2+ (g) + eMg2+ (g) + 7733 kJ ---> Mg3+ (g) + e- • Energy cost is very high to dip into a shell of lower n. This is why ox. no. = Group no. Trends in Ionization Energy Trend in Ionization Energy • IE increases across a period because Z* increases. – Metals lose electrons more easily than nonmetals. – Metals are good reducing agents. – Nonmetals lose electrons with difficulty. • IE decreases down a group because size increases – Reducing ability generally increases down the periodic table. Successive Ionization Energy Sample Problem • Using the periodic table only, rank the elements in each of the following sets in order of decreasing IE 1. 2. 3. 4. • Kr, He, Ar Sb, Te, Sn K, Ca, Rb I, Xe, Cs Rank in order of increasing IE 1. Sb, Sn, I 2. Sr, Ca, Ba Answers • Decreasing IE 1. 2. 3. 4. • He > Ar > Kr Te > Sb > Sn Ca > K > Rb Xe > I > Cs Rank in order of increasing IE 1. 2. Sn < Sb < I Ba < Sr < Ca Electron Affinity • A few elements GAIN electrons to form anions. • Electron affinity is the energy accompanying the addition of 1 mol electrons to 1 mol gaseous atoms or ions. A(g) + e- Ion-(g) ∆E = EA1 • In most cases energy is release when the first electron is added because it is attracted to the atom’s nuclear charge, thus EA1 is usually negative • Factors other than Zeff and atomic size affect electron affinities, so trends are not as regular as those the previous two properties • Despite irregularities, three key points emerge when examine ionization energy and electron affinity values: • Elements in groups 6A and especially 7A have high ionization energy and highly negative (exothermic) electron affinities. These elements lose electrons difficulty but attract them strongly so they form negative ions • Elements in groups 1A and 2A have low ionization energy and either slightly negative or positive (endothermic) electron affinities. These elements lose electron readily but attract them weakly, therefore in their ionic compounds they form positive ions • The noble gases, group 8A have very high ionization energy and highly positive electron affinities, therefore these elements do not tend to lose or gain electron. Electronegativity • Electronegativity is a measure of the relative ability of an element’s atoms to attract the shared electrons in a chemical bond. • Higher electronegativities mean a greater attraction for the electrons. • Fluorine is the highest with a value of 4.0 Bilangan oksidasi